The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study
et al., medRxiv, doi:10.1101/2020.09.09.20184143, Sep 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 423 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
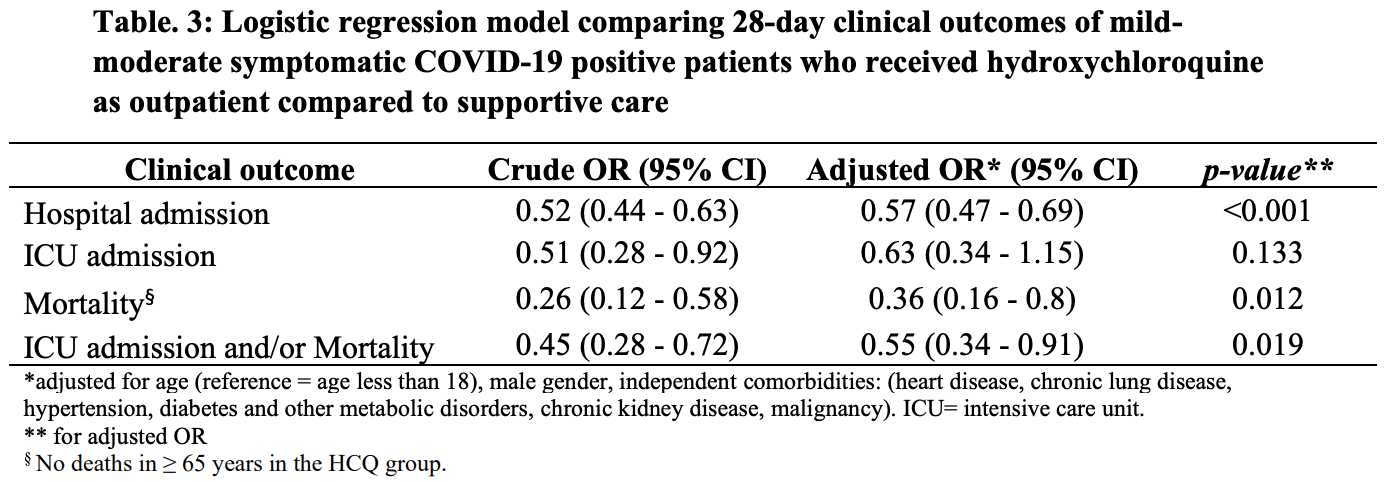
Observational prospective 5,541 patients, adjusted HCQ mortality odds ratio OR 0.36, p = 0.012. Adjusted hospitalization OR 0.57, p < 0.001. Zinc supplementation was used in all cases. Early treatment in ambulatory fever clinics in Saudi Arabia.
|
risk of death, 63.7% lower, RR 0.36, p = 0.01, treatment 7 of 1,817 (0.4%), control 54 of 3,724 (1.5%), NNT 94, adjusted per study, odds ratio converted to relative risk.
|
|
risk of death/ICU, 44.4% lower, RR 0.56, p = 0.02, treatment 21 of 1,817 (1.2%), control 95 of 3,724 (2.6%), adjusted per study, odds ratio converted to relative risk.
|
|
risk of ICU admission, 36.7% lower, RR 0.63, p = 0.13, treatment 14 of 1,817 (0.8%), control 56 of 3,724 (1.5%), adjusted per study, odds ratio converted to relative risk.
|
|
risk of hospitalization, 38.6% lower, RR 0.61, p < 0.001, treatment 171 of 1,817 (9.4%), control 617 of 3,724 (16.6%), NNT 14, adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sulaiman et al., 13 Sep 2020, prospective, Saudi Arabia, preprint, 22 authors, dosage 400mg bid day 1, 200mg bid days 2-5.
The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study
doi:10.1101/2020.09.09.20184143
BACKGROUND: Currently, there is no proven effective therapy nor vaccine for the treatment of SARS-CoV-2. Evidence regarding the potential benefit of early administration of hydroxychloroquine (HCQ) therapy in symptomatic patients with Coronavirus Disease (COVID-19) is not clear.
METHODS: This observational prospective cohort study took place in 238 ambulatory fever clinics in Saudi Arabia, which followed the Ministry of Health (MOH) COVID-19 treatment guideline. This guideline included multiple treatment options for COVID-19 based on the best available evidence at the time, among which was Hydroxychloroquine (HCQ). Patients with confirmed COVD-19 (by reverse transcriptase polymerase chain reaction (PCR) test) who presented to these clinics with mild to moderate symptoms during the period from 5-26 June 2020 were included in this study. Our study looked at those who received HCQ-based therapy along with supportive care (SC) and compared them to patients who received SC alone. The primary outcome was hospital admission within 28-days of presentation. The secondary outcome was a composite of intensive care admission (ICU) and/or mortality during the followup period. Outcome data were assessed through a follow-up telephonic questionnaire at day 28 and were further verified with national hospitalisation and mortality registries. Multiple logistic regression model was used to control for prespecified confounders.
RESULTS: Of the 7,892 symptomatic PCR-confirmed COVID-19 patients who visited the ambulatory fever clinics during the study period, 5,541 had verified clinical outcomes at day 28 (1,817 patients in the HCQ group vs 3,724 in the SC group). At baseline, patients who received HCQ therapy were more likely to be males who did not have hypertension or chronic lung disease compared to the SC group. No major differences were noted regarding other comorbid conditions. All patients were presenting with active complaints; however, the HCQ groups had higher rates of symptoms compared to the SC group (fever: 84% vs 66.3, headache: 49.8 vs 37.4, cough: 44.5 vs 35.6, respectively). Early HCQ-based therapy was associated with a lower hospital admission within 28-days compared to SC alone (9.4% compared to 16.6%, RRR 43%, p-value <0.001). The composite outcome of ICU admission and/or mortality at 28days was also lower in the HCQ group compared to the SC (1.2% compared to 2.6%, RRR 54%, p-value 0.001). Adjusting for age, gender, and major comorbid conditions, a multivariate logistic regression model showed a decrease in the odds of hospitalisation in patients who received HCQ compared to SC alone (adjusted OR 0.57 [95% CI 0.47-0.69], p-value <0.001). The composite outcome of ICU admission and/or mortality was also lower for the HCQ group All rights reserved. No reuse allowed without permission. perpetuity.
References
Arshad, Kilgore, Chaudhry, Jacobsen, treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalised with COVID-19, International Journal of Infectious Diseases
Boelart, Piette, Sperber, The potential place of chloroquine in the treatment of HIV-1-infected patients, Journal of Clinical Virology
Carlucci, Ahuja, Petrilli, Rajagopalan, Jones et al., Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalised COVID-19 patients, medRxiv, doi:10.1101/2020.05.02.20080036
Castelnuovo, Costanzo, Antinori, Berselli, Blandi et al., Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study, European Journal of Internal Medicine
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2019014
Chen, Hu, Zhang, Jiang, Han et al., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomised clinical trial. medRxiv, Available online
Derwand, Scholz, Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19?, Medical Hypotheses, doi:10.1016/j.mehy.2020.109815
Fesen, Kohn, Leteurtre, Inhibitors of human immunodeficiency virus integrase, Proc Natl Acad Sci
Frisk-Holmberg, Bergqvist, Englund, Chloroquine intoxication, Br. J. Clin. Pharm
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomised clinical trial, International Journal of Antimicrobial Agents
Geleris, Sun, Platt, Zucker, Baldwin et al., Observational study of hydroxychloroquine in hospitalised patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2012410
Guérin, Lévy, Thomas, Lardenois, Lacrosse et al., Azithromycin and hydroxychloroquine accelerate recovery of outpatients with mild/moderate COVID-19, doi:10.20944/preprints202005.0486.v1
Ip, Ahn, Zhou, Goy, Hansen et al., Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study, medRxiv, doi:10.1101/2020.08.20.20178772
Ip, Berry, Hansen, Goy, Pecora et al., Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients-An Observational Study, doi:10.1101/2020.05.21.20109207
Lagier, Million, Gautret, Colson, Sé et al., Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis, Travel Medicine andInfectiousDisease2020, doi:10.1016/j.tmaid.2020.101791
Li, Wang, Agostinis, Rabson, Melino et al., Is hydroxychloroquine beneficial for COVID-19 patients?, Cell death & disease
Mackenzie, Scherbel, Chloroquine and hydroxychloroquine in rheumatological therapy, Clin Rheum Dis
Mitjà, Corbacho-Monné, Ubals, Tebe, Peñafiel et al., Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomisedcontrolled trial, Clin Infect Dis, doi:10.1093/cid/ciaa10092020Jul16
Rosenberg, Dufort, Udo, Wilberschied, Kumar et al., Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state, JAMA, doi:10.1001/jama.2020.8630
Rynes, Ophthalmologic considerations in using antimalarials in the United States, Lupus
Savarino, Gennero, Sperber, The anti-HIV activity of chloroquine, Journal of Clinical Virology
Scholz, Derwand, Zelenko, COVID-19 Outpatients -Early Risk-Stratified Treatment with Zinc Plus Low Dose Hydroxychloroquine and Azithromycin: A Retrospective Case Series Study, Preprints, doi:10.20944/preprints202007.0025.v1
Stein, Gowda, Lifson, pH-independent HIV entry into CD4-positive cells via virus envelope fusion to the plasma membrane, Cell
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial, BMJ, doi:10.1136/bmj.m1849
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell research
Worldometers, Info, Corona Virus: Saudi Arabia
Wu, Wu, Liu, Yang, The SARS-CoV-2 outbreak: what we know, International Journal of Infectious Diseases
Zhou, Zhang, Qu, Coronavirus disease 2019 (COVID-19): a clinical update, Frontiers of medicine
DOI record:
{
"DOI": "10.1101/2020.09.09.20184143",
"URL": "http://dx.doi.org/10.1101/2020.09.09.20184143",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>BACKGROUND</jats:title><jats:p>Currently, there is no proven effective therapy nor vaccine for the treatment of SARS-CoV-2. Evidence regarding the potential benefit of early administration of hydroxychloroquine (HCQ) therapy in symptomatic patients with Coronavirus Disease (COVID-19) is not clear.</jats:p></jats:sec><jats:sec><jats:title>METHODS</jats:title><jats:p>This observational prospective cohort study took place in 238 ambulatory fever clinics in Saudi Arabia, which followed the Ministry of Health (MOH) COVID-19 treatment guideline. This guideline included multiple treatment options for COVID-19 based on the best available evidence at the time, among which was Hydroxychloroquine (HCQ). Patients with confirmed COVD-19 (by reverse transcriptase polymerase chain reaction (PCR) test) who presented to these clinics with mild to moderate symptoms during the period from 5-26 June 2020 were included in this study. Our study looked at those who received HCQ-based therapy along with supportive care (SC) and compared them to patients who received SC alone. The primary outcome was hospital admission within 28-days of presentation. The secondary outcome was a composite of intensive care admission (ICU) and/or mortality during the follow-up period. Outcome data were assessed through a follow-up telephonic questionnaire at day 28 and were further verified with national hospitalisation and mortality registries. Multiple logistic regression model was used to control for prespecified confounders.</jats:p></jats:sec><jats:sec><jats:title>RESULTS</jats:title><jats:p>Of the 7,892 symptomatic PCR-confirmed COVID-19 patients who visited the ambulatory fever clinics during the study period, 5,541 had verified clinical outcomes at day 28 (1,817 patients in the HCQ group vs 3,724 in the SC group). At baseline, patients who received HCQ therapy were more likely to be males who did not have hypertension or chronic lung disease compared to the SC group. No major differences were noted regarding other comorbid conditions. All patients were presenting with active complaints; however, the HCQ groups had higher rates of symptoms compared to the SC group (fever: 84% vs 66.3, headache: 49.8 vs 37.4, cough: 44.5 vs 35.6, respectively). Early HCQ-based therapy was associated with a lower hospital admission within 28-days compared to SC alone (9.4% compared to 16.6%, RRR 43%,<jats:italic>p-value</jats:italic><0.001). The composite outcome of ICU admission and/or mortality at 28-days was also lower in the HCQ group compared to the SC (1.2% compared to 2.6%, RRR 54%,<jats:italic>p-value</jats:italic>0.001). Adjusting for age, gender, and major comorbid conditions, a multivariate logistic regression model showed a decrease in the odds of hospitalisation in patients who received HCQ compared to SC alone (adjusted OR 0.57 [95% CI 0.47-0.69],<jats:italic>p-value <0.001</jats:italic>). The composite outcome of ICU admission and/or mortality was also lower for the HCQ group compared to the SC group controlling for potential confounders (adjusted OR 0.55 [95% CI 0.34-0.91],<jats:italic>p-value</jats:italic>0.019).</jats:p></jats:sec><jats:sec><jats:title>CONCLUSION</jats:title><jats:p>Early intervention with HCQ-based therapy in patients with mild to moderate symptoms at presentation is associated with lower adverse clinical outcomes among COVID-19 patients, including hospital admissions, ICU admission, and/or death.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
9,
13
]
]
},
"author": [
{
"affiliation": [],
"family": "Sulaiman",
"given": "Tarek",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-9646-5635",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mohana",
"given": "Abdulrhman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alawdah",
"given": "Laila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahmoud",
"given": "Nagla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassanein",
"given": "Mustafa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wani",
"given": "Tariq",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alfaifi",
"given": "Amel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alenazi",
"given": "Eissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Radwan",
"given": "Nashwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlKhalifah",
"given": "Nasser",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elkady",
"given": "Ehab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alanazi",
"given": "Manwer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alqahtani",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdullah",
"given": "Khalid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yousif",
"given": "Yousif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AboGazalah",
"given": "Fouad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Awwad",
"given": "Fuad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alabdulkareem",
"given": "Khaled",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlGhofaili",
"given": "Fahad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlJedai",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jokhdar",
"given": "Hani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alrabiah",
"given": "Fahad",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
13
]
],
"date-time": "2020-09-13T13:20:13Z",
"timestamp": 1600003213000
},
"deposited": {
"date-parts": [
[
2022,
11,
18
]
],
"date-time": "2022-11-18T02:29:08Z",
"timestamp": 1668738548000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
4,
3
]
],
"date-time": "2023-04-03T12:18:29Z",
"timestamp": 1680524309162
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2020,
9,
13
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.09.09.20184143",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
9,
13
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
9,
13
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021021605350676000_2020.09.09.20184143v1.1",
"unstructured": "Notification of 2019-nCoV infection. National Health Commission of the People’s Republic of China. http://www.nhc.gov.cn/xcs/yqfkdt/202002/18546da875d74445bb537ab014e7a1c6.shtml."
},
{
"article-title": "Coronavirus disease 2019 (COVID-19): a clinical update",
"first-page": "1",
"journal-title": "Frontiers of medicine",
"key": "2021021605350676000_2020.09.09.20184143v1.2",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.03.004",
"article-title": "The SARS-CoV-2 outbreak: what we know",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "International Journal of Infectious Diseases",
"key": "2021021605350676000_2020.09.09.20184143v1.3",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1055/a-1347-6070",
"doi-asserted-by": "crossref",
"key": "2021021605350676000_2020.09.09.20184143v1.4",
"unstructured": "Di Castelnuovo A , Costanzo S , Antinori A , Berselli N , Blandi L , Bruno R , et al. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. European Journal of Internal Medicine. 2020 Aug 25. Article in press"
},
{
"article-title": "Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis",
"first-page": "101791",
"journal-title": "Travel Medicine andInfectiousDisease",
"key": "2021021605350676000_2020.09.09.20184143v1.5",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.6"
},
{
"DOI": "10.1016/S1386-6532(00)00139-6",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.7"
},
{
"DOI": "10.1016/S0307-742X(21)00317-9",
"article-title": "Chloroquine and hydroxychloroquine in rheumatological therapy",
"doi-asserted-by": "crossref",
"first-page": "545",
"journal-title": "Clin Rheum Dis",
"key": "2021021605350676000_2020.09.09.20184143v1.8",
"volume": "6",
"year": "1980"
},
{
"DOI": "10.1177/096120339600500117",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.9"
},
{
"DOI": "10.1016/S1386-6532(00)00140-2",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.10"
},
{
"DOI": "10.1016/0092-8674(87)90542-3",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.11"
},
{
"DOI": "10.1073/pnas.90.6.2399",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.12"
},
{
"key": "2021021605350676000_2020.09.09.20184143v1.13"
},
{
"DOI": "10.20944/preprints202007.0025.v1",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.14"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.15"
},
{
"key": "2021021605350676000_2020.09.09.20184143v1.16"
},
{
"DOI": "10.1101/2020.05.21.20109207",
"doi-asserted-by": "crossref",
"key": "2021021605350676000_2020.09.09.20184143v1.17",
"unstructured": "Ip A , Berry DA , Hansen E , Goy AH , Pecora AL , Sinclaire BA , et al. Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients-An Observational Study. medRxiv. 2020 Available online Jan 1: https://doi.org/10.1101/2020.05.21.20109207."
},
{
"DOI": "10.1056/NEJMoa2012410",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.18"
},
{
"DOI": "10.1001/jama.2020.8630",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.19"
},
{
"article-title": "Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial",
"first-page": "1849",
"journal-title": "BMJ",
"key": "2021021605350676000_2020.09.09.20184143v1.20",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2019014",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.21"
},
{
"DOI": "10.1016/j.mehy.2020.109815",
"doi-asserted-by": "crossref",
"key": "2021021605350676000_2020.09.09.20184143v1.22",
"unstructured": "Derwand R , Scholz M . Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Medical Hypotheses 2020 doi: https://doi.org/10.1016/j.mehy.2020.109815"
},
{
"DOI": "10.1101/2020.05.02.20080036",
"doi-asserted-by": "crossref",
"key": "2021021605350676000_2020.09.09.20184143v1.23",
"unstructured": "Carlucci P , Ahuja T , Petrilli CM , Rajagopalan H , Jones S , Rahimian J . Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalised COVID-19 patients. medRxiv. doi: https://doi.org/10.1101/2020.05.02.20080036"
},
{
"DOI": "10.1111/j.1365-2125.1983.tb01540.x",
"article-title": "Chloroquine intoxication",
"doi-asserted-by": "crossref",
"first-page": "502",
"journal-title": "Br. J. Clin. Pharm",
"key": "2021021605350676000_2020.09.09.20184143v1.24",
"volume": "15",
"year": "1983"
},
{
"key": "2021021605350676000_2020.09.09.20184143v1.25",
"unstructured": "Saudi Ministry of Health Protocol for patients suspected/confirmed with COVID-19. https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf 2020 Version 1.6 May 24th."
},
{
"key": "2021021605350676000_2020.09.09.20184143v1.26",
"unstructured": "The Saudi Centre for Disease Prevention and Control. Information about Coronavirus Disease: COVID-19. 2020. Available at: <https://covid19.cdc.gov.sa/>"
},
{
"key": "2021021605350676000_2020.09.09.20184143v1.27",
"unstructured": "Worldometers.info. Corona Virus: Saudi Arabia. Available at: https://www.worldometers.info/coronavirus/country/saudi-arabia/ (accessed August 27, 2020)."
},
{
"article-title": "Is hydroxychloroquine beneficial for COVID-19 patients",
"first-page": "1",
"journal-title": "Cell death & disease",
"key": "2021021605350676000_2020.09.09.20184143v1.28",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1009",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.29"
},
{
"DOI": "10.20944/preprints202005.0486.v1",
"doi-asserted-by": "publisher",
"key": "2021021605350676000_2020.09.09.20184143v1.30"
},
{
"DOI": "10.1101/2020.08.20.20178772",
"doi-asserted-by": "crossref",
"key": "2021021605350676000_2020.09.09.20184143v1.31",
"unstructured": "Ip A , Ahn J , Zhou Y , Goy AH , Hansen E , Pecora AL , Sinclaire BA , Bednarz U , Marafelias M , Mathura S , Sawczuk IS . Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study. medRxiv. 2020 Jan 1. doi: https://doi.org/10.1101/2020.08.20.20178772"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.09.09.20184143"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study",
"type": "posted-content"
}
