Early Treatment of Favipiravir in COVID-19 Patients Without Pneumonia: A Multicentre, Open-Labelled, Randomized Control Study
et al., medRxiv, doi:10.1101/2022.06.06.22275902, TCTR20200514001, Jun 2022
RCT 93 patients in Thailand showing significantly faster clinical improvement with favipiravir treatment. 1800mg favipiravir bid day 1, 800mg bid 5-14 days until PCR-.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
time to clinical improvement, 63.9% lower, HR 0.36, p < 0.001, treatment 62, control 31, inverted to make HR<1 favor treatment, primary outcome.
|
|
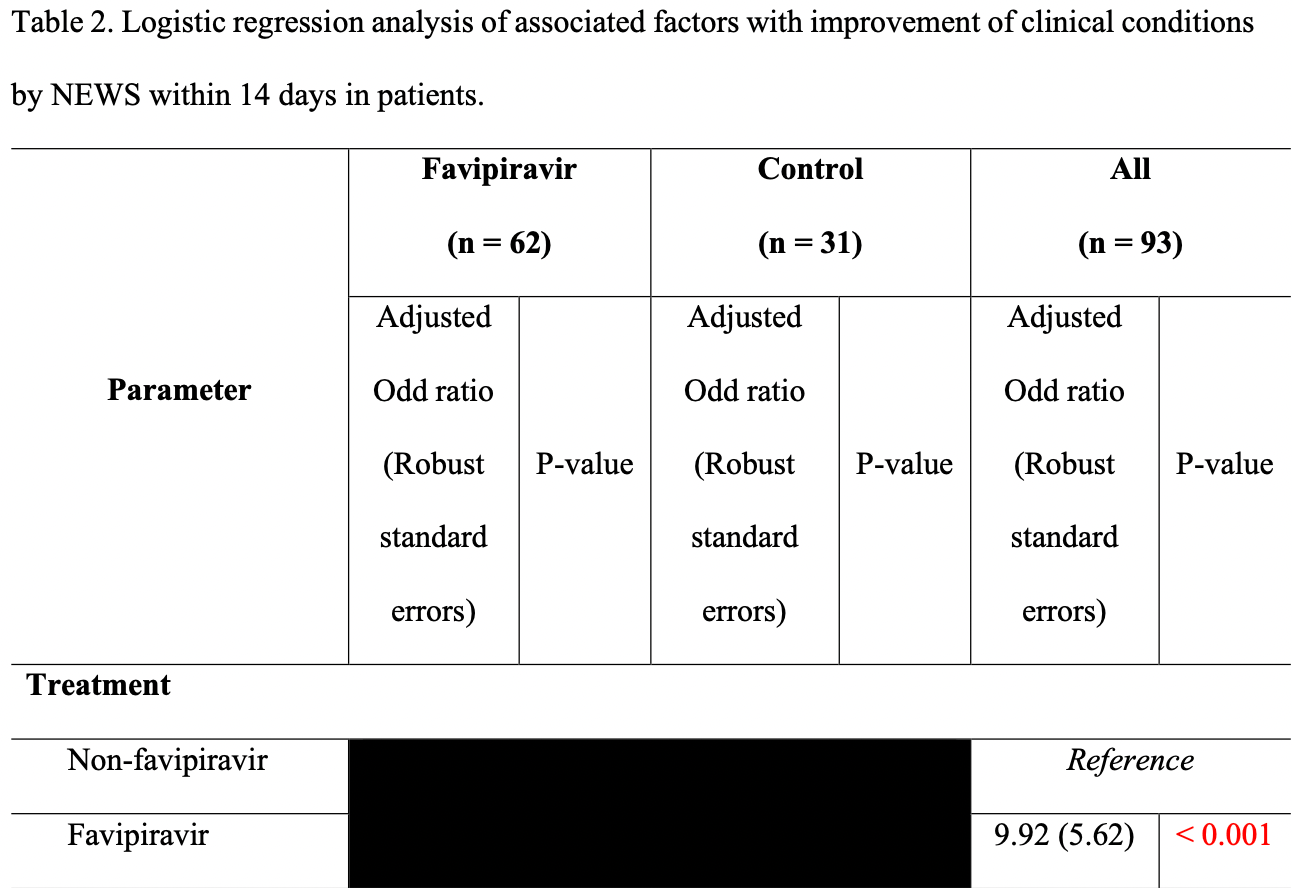
clinical improvement, 89.9% lower, OR 0.10, p < 0.001, treatment 62, control 31, inverted to make OR<1 favor treatment, logistic regression, day 14, RR approximated with OR.
|
|
risk of mild pneumonia, 42.9% lower, RR 0.57, p = 0.25, treatment 8 of 62 (12.9%), control 7 of 31 (22.6%), NNT 10.
|
|
risk of no viral clearance, 4.2% higher, HR 1.04, p = 0.87, treatment 62, control 31, adjusted per study, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Sirijatuphat et al., 8 Jun 2022, Randomized Controlled Trial, Thailand, peer-reviewed, 9 authors, study period December 2020 - July 2021, trial TCTR20200514001.
Early Treatment of Favipiravir in COVID-19 Patients Without Pneumonia: A Multicentre, Open-Labelled, Randomized Control Study
doi:10.1101/2022.06.06.22275902
We investigated Favipiravir (FPV) efficacy in mild cases of COVID-19 without pneumonia and its effects towards viral clearance, clinical condition, and risk of COVID-19 pneumonia development. PCR-confirmed SARS-CoV-2-infected patients without pneumonia were enrolled (2:1) within 10 days of symptomatic onset into FPV and control arms. The former received 1800 mg FPV twice-daily (BID) on Day 1 and 800 mg BID 5-14 days thereafter until negative viral detection, while the latter received supportive care only. The primary endpoint was time to clinical improvement, which was defined by a reduced National Early Warning Score (NEWS) or score of <1. 62 patients (41 female) comprised the FPV arm (median age: 32 years, median BMI: 22 kg/m²) and 31 patients (19 female) comprised the control arm (median age: 28 years, median BMI: 22 kg/m². The median time to sustained clinical improvement by NEWS was 2 vs 14 days for FPV and control arms respectively (adjusted hazard ratio (aHR) of 2.77, 95% CI 1.57-4.88, P <0.001). The FPV arm also had significantly higher likelihoods of clinical improvement within 14 days after enrolment by NEWS (79% vs 32% respectively, P <0.001), particularly female patients (aOR 6.35, 95% CI 1.49-27.07, P <0.001). 8 (12.9%) and 7 (22.6%) patients in FPV and control arms developed mild pneumonia at a median (range) 6.5 (1-13) and 7 (1-13) days after treatment, respectively (P = 0.316); all recovered well without complications. We can conclude that early treatment of FPV in symptomatic COVID-19 patients without pneumonia was associated with faster clinical improvement.
Declaration of Interest Statement AO is a Director of Tandem Nano Ltd and co-inventor of patents relating to drug delivery. AO
References
Ader, Bouscambert-Duchamp, Hites, Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis
Agrawal, Raju, Udwadia, Favipiravir: A new and emerging antiviral option in COVID-19, Med J Armed Forces India
Alene, Yismaw, Assemie, Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis, PLoS One
Bai, Mu, Kargbo, Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated With Favipiravir (T-705)-Sierra Leone, 2014, Clin Infect Dis
Baranovich, Wong, Armstrong, T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro, J Virol
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, New England Journal of Medicine
Brophy, Molnupiravir's authorisation was premature
Cai, Yang, Liu, Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Chen, Chao, Lai, Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients, J Infect
Chuah, Chow, Hor, Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial, Clin Infect Dis
Deng, Yang, Yang, Evaluation of favipiravir in the treatment of COVID-19 based on the real-world, Expert Rev Anti Infect Ther
Do, National Early Warning Score, National Clinical Guideline
Doi, Hibino, Hase, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob Agents Chemother
Eroglu, Toprak, Overview of favipiravir and remdesivir treatment for COVID-19
Fischer, Eron, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Fujifilm Toyama, Co, Notice of The New Drug Application Approval of "AVIGAN® Tablet 200mg
Fujii, Ibe, Ishigo, Early favipiravir treatment was associated with early defervescence in non-severe COVID-19 patients, Journal of Infection and Chemotherapy
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Ghasemnejad-Berenji, Pashapour, Favipiravir and COVID-19: A Simplified Summary, Drug Res (Stuttg)
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Gowen, Wong, Jung, In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections, Antimicrob Agents Chemother
Hassanipour, Arab-Zozani, Amani, The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep
Holubar, Subramanian, Purington, Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial, Clin Infect Dis
Ivashchenko, Dmitriev, Vostokova, AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin Infect Dis
Joshi, Parkar, Ansari, Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Kelleni, Tocilizumab, Favipiravir, and Dexamethasone Repurposed for COVID-19: a Comprehensive Clinical and Pharmacovigilant Reassessment, SN Compr Clin Med
Ko, Jeon, Ryu, Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells, J Med Virol
Kumari, Rawat, Saha, Pipeline Pharmacological Therapies in Clinical Trial for COVID-19 Pandemic: a Recent Update, Curr Pharmacol Rep
Kutsuna, Coronavirus disease
Lu, Chen, Lee, Potential therapeutic agents against COVID-19: What we know so far, J Chin Med Assoc
Manabe, Kambayashi, Akatsu, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis
Manosuthi, Jeungsmarn, Okada, Nasopharyngeal SARS-CoV-2 Viral Load Response among COVID-19 Patients Receiving Favipiravir, Jpn J Infect Dis
Mendenhall, Russell, Smee, Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic Fever, PLoS Negl Trop Dis
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med
Oestereich, Lüdtke, Wurr, Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model, Antiviral Res
Organization, WHO Coronavirus (COVID-19) Dashboard
Pertinez, Rajoli, Khoo, Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5'-triphosphate exposure to support posology for SARS-CoV-2, J Antimicrob Chemother
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, J Virus Erad
Pongpirul, Wiboonchutikul, Charoenpong, Clinical course and potential predictive factors for pneumonia of adult patients with Coronavirus Disease 2019 (COVID-19): A retrospective observational analysis of 193 confirmed cases in Thailand, PLoS Negl Trop Dis
Prakash, Singh, Kaur, Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients, Indian J Pharmacol
Prasithsirikul, Pongpirul, Sakornsakolpat, Adjunctive favipiravir for severe COVID-19: a retrospective observational study of the first 41 patients in Thailand, Asian Biomedicine
Reddy, Lai, Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options, Chembiochem
Sawanpanyalert, Sirijatuphat, Sangsayunh, Assessement of outcomes following implementation of antiviral treatment guidelines for COVID-19 during the first wave in Thailand, Southeast Asian Journal of Tropical Medicine and Public Health
Seneviratne, Abeysuriya, Mel, Favipiravir in COVID-19, International Journal of Progressive Sciences and Technologies
Sheahan, Sims, Zhou, An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Sci Transl Med
Shinada, Sato, Moriyama, Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir, Viruses
Shrestha, Budhathoki, Khadka, Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virol J
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, Bmj
Srinivas, Sacha, Koval, Antivirals for COVID-19, Cleaveland Clinical Journal of Medicine
Thorlund, Sheldrick, Mills, Molnupiravir for Covid-19 in Nonhospitalized Patients
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNAdependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wei, Moradkhani, Hezaveh, Evaluating the Treatment with Favipiravir in Patients Infected by COVID-19: A Systematic Review and Meta-analysis International Journal of Scientific Research in Dental, and Medical Sciences
Yanai, Favipiravir: A Possible Pharmaceutical Treatment for COVID-19, Journal of Endocrinology & Metabolism
Özlüşen, Kozan, Akcan, Effectiveness of favipiravir in COVID-19: a live systematic review, Eur J Clin Microbiol Infect Dis
DOI record:
{
"DOI": "10.1101/2022.06.06.22275902",
"URL": "http://dx.doi.org/10.1101/2022.06.06.22275902",
"abstract": "<jats:title>Abstract</jats:title><jats:p>We investigated Favipiravir (FPV) efficacy in mild cases of COVID-19 without pneumonia and its effects towards viral clearance, clinical condition, and risk of COVID-19 pneumonia development. PCR-confirmed SARS-CoV-2-infected patients without pneumonia were enrolled (2:1) within 10 days of symptomatic onset into FPV and control arms. The former received 1800 mg FPV twice-daily (BID) on Day 1 and 800 mg BID 5-14 days thereafter until negative viral detection, while the latter received supportive care only. The primary endpoint was time to clinical improvement, which was defined by a reduced National Early Warning Score (NEWS) or score of ≤1. 62 patients (41 female) comprised the FPV arm (median age: 32 years, median BMI: 22 kg/m²) and 31 patients (19 female) comprised the control arm (median age: 28 years, median BMI: 22 kg/m². The median time to sustained clinical improvement by NEWS was 2 vs 14 days for FPV and control arms respectively (adjusted hazard ratio (aHR) of 2.77, 95% CI 1.57-4.88, <jats:italic>P</jats:italic> <0.001). The FPV arm also had significantly higher likelihoods of clinical improvement within 14 days after enrolment by NEWS (79% vs 32% respectively, <jats:italic>P</jats:italic> <0.001), particularly female patients (aOR 6.35, 95% CI 1.49-27.07, <jats:italic>P</jats:italic> <0.001). 8 (12.9%) and 7 (22.6%) patients in FPV and control arms developed mild pneumonia at a median (range) 6.5 (1-13) and 7 (1-13) days after treatment, respectively (<jats:italic>P</jats:italic> = 0.316); all recovered well without complications. We can conclude that early treatment of FPV in symptomatic COVID-19 patients without pneumonia was associated with faster clinical improvement.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
6,
8
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1486-5550",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sirijatuphat",
"given": "Rujipas",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2583-9390",
"affiliation": [],
"authenticated-orcid": false,
"family": "Manosuthi",
"given": "Weerawat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4785-2716",
"affiliation": [],
"authenticated-orcid": false,
"family": "Niyomnaitham",
"given": "Suvimol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Copeland",
"given": "Katherine K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3811-0267",
"affiliation": [],
"authenticated-orcid": false,
"family": "Charoenpong",
"given": "Lantharita",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1150-2597",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rattanasompattikul",
"given": "Manoch",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7555-1056",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mahasirimongkol",
"given": "Surakameth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0140-4600",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chokephaibulkit",
"given": "Kulkanya",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
8
]
],
"date-time": "2022-06-08T15:00:11Z",
"timestamp": 1654700411000
},
"deposited": {
"date-parts": [
[
2022,
6,
10
]
],
"date-time": "2022-06-10T09:46:15Z",
"timestamp": 1654854375000
},
"group-title": "Pharmacology and Therapeutics",
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T04:35:24Z",
"timestamp": 1711600524335
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2022,
6,
8
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.06.06.22275902",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
6,
8
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
6,
8
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2022061002451014000_2022.06.06.22275902v1.1",
"unstructured": "Organization WH. WHO Coronavirus (COVID-19) Dashboard 2022. Available from: https://covid19.who.int/"
},
{
"DOI": "10.35772/ghm.2020.01031",
"article-title": "Coronavirus disease 2019 (COVID-19): research progress and clinical practice",
"doi-asserted-by": "crossref",
"first-page": "78",
"issue": "2",
"journal-title": "Glob Health Med",
"key": "2022061002451014000_2022.06.06.22275902v1.2",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2021.01.015",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.3"
},
{
"article-title": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study",
"first-page": "1192",
"issue": "10",
"journal-title": "Engineering (Beijing)",
"key": "2022061002451014000_2022.06.06.22275902v1.4",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1371/journal.pntd.0008806",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.5"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"article-title": "Role of favipiravir in the treatment of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Int J Infect Dis",
"key": "2022061002451014000_2022.06.06.22275902v1.6",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0249090",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.7"
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.8",
"unstructured": "Doi Y , Hibino M , Hase R , et al. A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19. Antimicrob Agents Chemother. 2020 Nov 17;64(12)."
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.9"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.10"
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.11",
"unstructured": "Eroglu E , Toprak C. Overview of favipiravir and remdesivir treatment for COVID-19. International Journal of Pharmaceutical Sciences and Research. 2021:1950–1957."
},
{
"DOI": "10.1007/s40495-020-00226-5",
"article-title": "Pipeline Pharmacological Therapies in Clinical Trial for COVID-19 Pandemic: a Recent Update",
"doi-asserted-by": "crossref",
"first-page": "228",
"issue": "5",
"journal-title": "Curr Pharmacol Rep",
"key": "2022061002451014000_2022.06.06.22275902v1.12",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"article-title": "Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "489",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "2022061002451014000_2022.06.06.22275902v1.13",
"volume": "21",
"year": "2021"
},
{
"article-title": "Evaluating the Treatment with Favipiravir in Patients Infected by COVID-19",
"first-page": "87",
"issue": "3",
"journal-title": "A Systematic Review and Meta-analysis International Journal of Scientific Research in Dental and Medical Sciences",
"key": "2022061002451014000_2022.06.06.22275902v1.14",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1007/s42399-021-00824-4",
"article-title": "Tocilizumab, Remdesivir, Favipiravir, and Dexamethasone Repurposed for COVID-19: a Comprehensive Clinical and Pharmacovigilant Reassessment",
"doi-asserted-by": "crossref",
"first-page": "919",
"issue": "4",
"journal-title": "SN Compr Clin Med",
"key": "2022061002451014000_2022.06.06.22275902v1.15",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26397",
"article-title": "Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells",
"doi-asserted-by": "crossref",
"first-page": "1403",
"issue": "3",
"journal-title": "J Med Virol",
"key": "2022061002451014000_2022.06.06.22275902v1.16",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1002/cbic.202000595",
"article-title": "Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options",
"doi-asserted-by": "crossref",
"first-page": "939",
"issue": "6",
"journal-title": "Chembiochem",
"key": "2022061002451014000_2022.06.06.22275902v1.17",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.18"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.19"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "New England Journal of Medicine",
"key": "2022061002451014000_2022.06.06.22275902v1.20",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201612",
"article-title": "Molnupiravir for Covid-19 in Nonhospitalized Patients",
"doi-asserted-by": "crossref",
"first-page": "e32",
"issue": "13",
"journal-title": "New England Journal of Medicine",
"key": "2022061002451014000_2022.06.06.22275902v1.21",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1136/bmj.o443",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.22",
"unstructured": "Brophy JM . Molnupiravir’s authorisation was premature. British Medical Journal Publishing Group; 2022."
},
{
"article-title": "Favipiravir in COVID-19",
"first-page": "143",
"issue": "2",
"journal-title": "International Journal of Progressive Sciences and Technologies",
"key": "2022061002451014000_2022.06.06.22275902v1.23",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.24"
},
{
"DOI": "10.1016/j.mjafi.2020.08.004",
"article-title": "Favipiravir: A new and emerging antiviral option in COVID-19",
"doi-asserted-by": "crossref",
"first-page": "370",
"issue": "4",
"journal-title": "Med J Armed Forces India",
"key": "2022061002451014000_2022.06.06.22275902v1.25",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.26"
},
{
"DOI": "10.1093/cid/ciaa1176",
"article-title": "AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "531",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "2022061002451014000_2022.06.06.22275902v1.27",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.14740/jem645",
"article-title": "Favipiravir: A Possible Pharmaceutical Treatment for COVID-19",
"doi-asserted-by": "crossref",
"first-page": "33",
"issue": "2",
"journal-title": "Journal of Endocrinology & Metabolism",
"key": "2022061002451014000_2022.06.06.22275902v1.28",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciac312",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.29",
"unstructured": "Holubar M , Subramanian A , Purington N , et al. Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial. Clin Infect Dis. 2022 Apr 21."
},
{
"DOI": "10.3390/v14040670",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.30",
"unstructured": "Shinada K , Sato T , Moriyama S , et al. Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir. Viruses. 2022 Mar 24;14(4)."
},
{
"DOI": "10.1007/s10096-021-04307-1",
"article-title": "Effectiveness of favipiravir in COVID-19: a live systematic review",
"doi-asserted-by": "crossref",
"first-page": "2575",
"issue": "12",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "2022061002451014000_2022.06.06.22275902v1.31",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.32"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"doi-asserted-by": "crossref",
"first-page": "11022",
"issue": "1",
"journal-title": "Sci Rep",
"key": "2022061002451014000_2022.06.06.22275902v1.33",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.34"
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.35",
"unstructured": "Fujifilm Toyama Chemical Co. L. Notice of The New Drug Application Approval of “AVIGAN® Tablet 200mg” in Japan for the Anti-influenza Virus Drug 2014. Available from: https://www.cdc.gov.tw/File/Get/ht8jUiB_MI-aKnlwstwzvw"
},
{
"DOI": "10.1515/abm-2020-0016",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.36",
"unstructured": "Prasithsirikul W , Pongpirul K , Sakornsakolpat P , et al. Adjunctive favipiravir for severe COVID-19: a retrospective observational study of the first 41 patients in Thailand. Asian Biomedicine. 2020;14(3)."
},
{
"DOI": "10.1371/journal.pntd.0001342",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.37"
},
{
"DOI": "10.1016/j.antiviral.2014.02.014",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.38"
},
{
"DOI": "10.1128/AAC.00356-07",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.39"
},
{
"DOI": "10.1093/cid/ciw571",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.40"
},
{
"DOI": "10.1097/JCMA.0000000000000318",
"article-title": "Potential therapeutic agents against COVID-19: What we know so far",
"doi-asserted-by": "crossref",
"first-page": "534",
"issue": "6",
"journal-title": "J Chin Med Assoc",
"key": "2022061002451014000_2022.06.06.22275902v1.41",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1128/JVI.02346-12",
"doi-asserted-by": "publisher",
"key": "2022061002451014000_2022.06.06.22275902v1.42"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.43",
"unstructured": "Sheahan TP , Sims AC , Zhou S , et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020 Apr 29;12(541)."
},
{
"article-title": "Assessement of outcomes following implementation of antiviral treatment guidelines for COVID-19 during the first wave in Thailand",
"first-page": "572",
"issue": "4",
"journal-title": "Southeast Asian Journal of Tropical Medicine and Public Health",
"key": "2022061002451014000_2022.06.06.22275902v1.44",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1016/j.jiac.2021.04.013",
"article-title": "Early favipiravir treatment was associated with early defervescence in non-severe COVID-19 patients",
"doi-asserted-by": "crossref",
"first-page": "1051",
"issue": "7",
"journal-title": "Journal of Infection and Chemotherapy",
"key": "2022061002451014000_2022.06.06.22275902v1.45",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1055/a-1296-7935",
"article-title": "Favipiravir and COVID-19: A Simplified Summary",
"doi-asserted-by": "crossref",
"first-page": "166",
"issue": "3",
"journal-title": "Drug Res (Stuttg)",
"key": "2022061002451014000_2022.06.06.22275902v1.46",
"volume": "71",
"year": "2021"
},
{
"DOI": "10.3949/ccjm.87a.ccc030",
"doi-asserted-by": "crossref",
"key": "2022061002451014000_2022.06.06.22275902v1.47",
"unstructured": "Srinivas P , Sacha G , Koval C. Antivirals for COVID-19. Cleaveland Clinical Journal of Medicine. 2020."
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.48",
"unstructured": "Medical Research Foundation T. Thai Clinical Trials Registry 2021. Available from: https://thaiclinicaltrials.org/"
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.49",
"unstructured": "Health Do. National Early Warning Score: National Clinical Guideline No. 1 2013."
},
{
"DOI": "10.4103/ijp.ijp_998_20",
"article-title": "Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients",
"doi-asserted-by": "crossref",
"first-page": "414",
"issue": "5",
"journal-title": "Indian J Pharmacol",
"key": "2022061002451014000_2022.06.06.22275902v1.50",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.7883/yoken.JJID.2020.827",
"article-title": "Nasopharyngeal SARS-CoV-2 Viral Load Response among COVID-19 Patients Receiving Favipiravir",
"doi-asserted-by": "crossref",
"first-page": "416",
"issue": "5",
"journal-title": "Jpn J Infect Dis",
"key": "2022061002451014000_2022.06.06.22275902v1.51",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1080/14787210.2022.2012155",
"article-title": "Evaluation of favipiravir in the treatment of COVID-19 based on the real-world",
"doi-asserted-by": "crossref",
"first-page": "555",
"issue": "4",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "2022061002451014000_2022.06.06.22275902v1.52",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"doi-asserted-by": "crossref",
"first-page": "eabl7430",
"issue": "628",
"journal-title": "Sci Transl Med",
"key": "2022061002451014000_2022.06.06.22275902v1.53",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"doi-asserted-by": "crossref",
"first-page": "209",
"issue": "2",
"journal-title": "Lancet Infect Dis",
"key": "2022061002451014000_2022.06.06.22275902v1.54",
"volume": "22",
"year": "2022"
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.55",
"unstructured": "Chuah CH , Chow TS , Hor CP , et al. Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial. Clin Infect Dis. 2021 Nov 19."
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.56",
"unstructured": "35 generic manufacturers sign agreements with MPP to produce low-cost, generic versions of Pfizer’s oral COVID-19 treatment nirmatrelvir in combination with ritonavir for supply in 95 low-and middle-income countries [Internet]. Medicines Patent Pool; 2022. Available from: https://medicinespatentpool.org/news-publications-post/35-generic-manufacturers-sign-agreements-with-mpp-to-produce-low-cost-generic-versions-of-pfizers-oral-covid-19-treatment-nirmatrelvir-in-combination-with-ritonavir-for-supply-in-95-low-and"
},
{
"key": "2022061002451014000_2022.06.06.22275902v1.57",
"unstructured": "27 generic manufacturers sign agreements with MPP to produce low-cost versions of COVID-19 antiviral medication molnupiravir for supply in 105 low-and-middle-income countries [Internet]. Medicines Patent Pool; 2022. Available from: https://medicinespatentpool.org/news-publications-post/27-generic-manufacturers-sign-agreements-with-mpp-to-produce-molnupiravir"
},
{
"DOI": "10.1093/jac/dkab135",
"article-title": "Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5’-triphosphate exposure to support posology for SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "2121",
"issue": "8",
"journal-title": "J Antimicrob Chemother",
"key": "2022061002451014000_2022.06.06.22275902v1.58",
"volume": "76",
"year": "2021"
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.06.06.22275902"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Early Treatment of Favipiravir in COVID-19 Patients Without Pneumonia: A Multicentre, Open-Labelled, Randomized Control Study",
"type": "posted-content"
}