Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China
et al., medRxiv, doi:10.1101/2023.05.10.23289325, May 2023
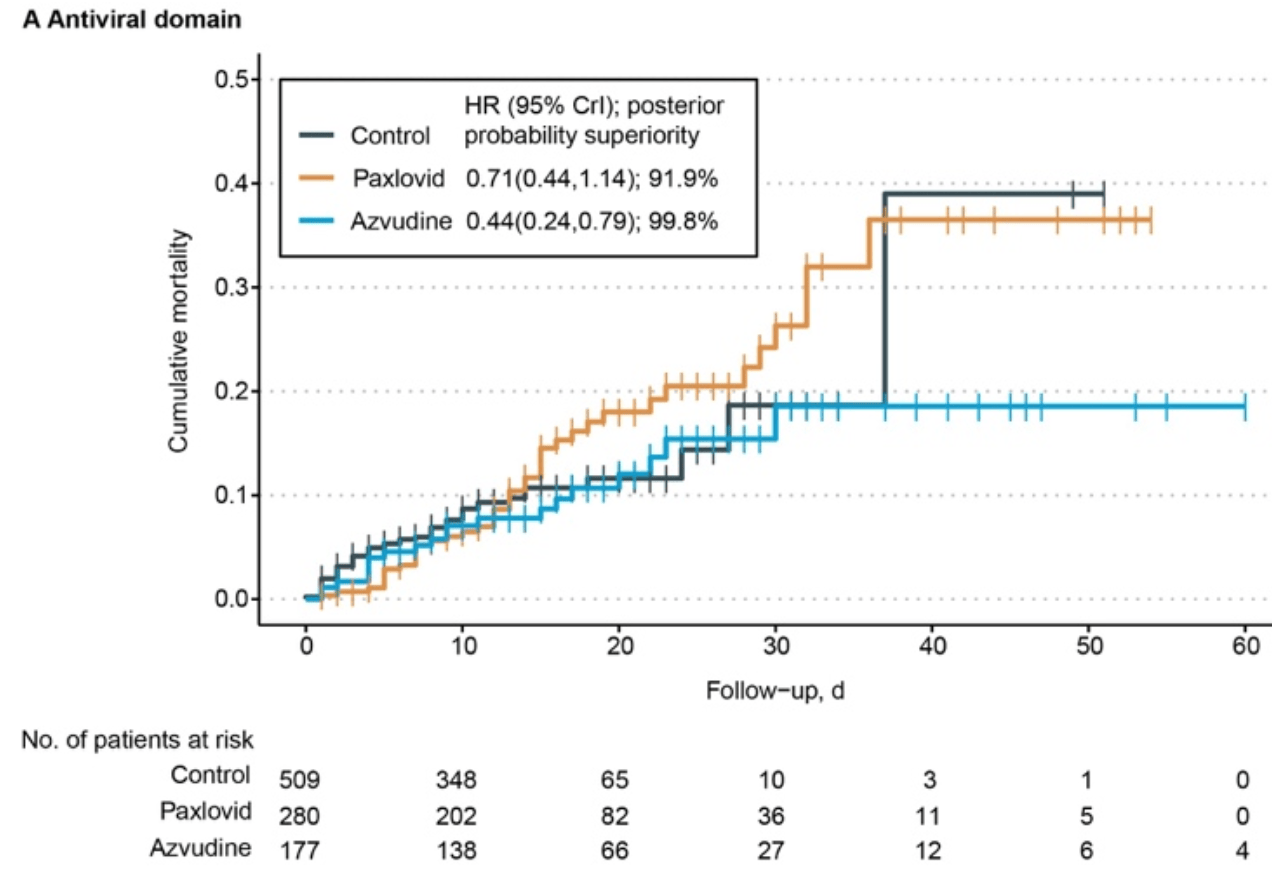
Retrospective 1,082 hospitalized COVID-19 patients in China, showing lower mortality and worse quality of life with paxlovid.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
risk of death, 29.0% lower, HR 0.71, p = 0.16, treatment 280, control 802, day 60.
|
|
relative HRQoL score, 28.3% worse, RR 1.28, p < 0.001, treatment mean 0.46 (±0.42) n=237, control mean 0.59 (±0.41) n=456, day 60.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Shao et al., 14 May 2023, retrospective, China, peer-reviewed, 9 authors, study period 8 December, 2022 - 9 February, 2023.
Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China
doi:10.1101/2023.05.10.23289325
All rights reserved. No reuse allowed without permission. (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
Author contributions
Conflict of Interest All authors in the article declared that they have no competing interests.
Ethical approval Statement This study was approved by the Ethics Committee of Jiading District Central Hospital affiliated to Shanghai University of Medicine and Health Sciences (Approval code 2023K15)
References
Albumin, None
Bellet, Renga, Pariano, Stincardini, Onofrio et al., COVID-19 and beyond: Reassessing the role of thymosin alpha1 in lung infections, Int Immunopharmacol
Bolek, Samos, Jurica, Stanciakova, Pec et al., COVID-19 and the Response to Antiplatelet Therapy, J Clin Med
Cilloniz, Motos, Castaneda, Gabarrus, Barbe et al., Remdesivir and survival outcomes in critically ill patients with COVID-19: A multicentre observational cohort study, J Infect
Committee For The R-Capi, Estcourt, Turgeon, Mcquilten, Mcverry et al., Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial, JAMA
Committee For The R-Capi, Higgins, Berry, Lorenzi, Murthy et al., Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized Clinical Trial, JAMA
Crp, None
Dyer, Covid-19: China stops counting cases as models predict a million or more deaths, BMJ
Ely, Ramanan, Kartman, De Bono, Liao et al., Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial, Lancet Respir Med
Ferritin, None
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Huang, Li, Gu, Zhang, Ren et al., Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study, Lancet Respir Med
Huang, Yao, Gu, Wang, Ren et al., 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study, Lancet
Investigators, Bradbury, Lawler, Stanworth, Mcverry et al., Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial, JAMA
Investigators, Investigators, Investigators, Goligher, Bradbury et al., Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19, N Engl J Med
Ioannidis, Zonta, Levitt, Estimates of COVID-19 deaths in Mainland China after abandoning zero COVID policy, Eur J Clin Invest
Iu/L, None
Iu/L, None
Iu/L, None
Jimenez-Mora, Varela, Meneses-Echavez, Bidonde, Angarita-Fonseca et al., Patient-important outcomes reported in randomized controlled trials of pharmacologic treatments for COVID-19: a protocol of a META-epidemiological study, Syst Rev
Liu, Pan, Hu, Wu, Wang et al., Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells, Clin Infect Dis
Liu, Pan, Zhang, Li, Ma et al., Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study, Lancet Reg Health West Pac
Liu, Zhang, Lu, Li, Wu et al., Benefits of high-dose intravenous immunoglobulin on mortality in patients with severe COVID-19: An updated systematic review and meta-analysis, Front Immunol
Luo, Liu, Li, Guan, Rand-Hendriksen, Estimating an EQ-5D-5L Value Set for China
Martinez-Guerra, Gonzalez-Lara, Montes, Tamez-Torres, Fierro et al., Outcomes of patients with severe and critical COVID-19 treated with dexamethasone: a prospective cohort study, Emerg Microbes Infect
Plt, None
Salehi, Mehni, Akbarian, Ghazi, Rad et al., The outcome of using intravenous immunoglobulin (IVIG) in critically ill COVID-19 patients': a retrospective, multi-centric cohort study, Eur J Med Res
Scr, None
Shao, Fan, Hu, Zhang, Lee et al., Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant, Vaccines (Basel)
Spaetgens, Nagy, Cate, Antiplatelet Therapy in Patients With COVID-19-More Is Less?, JAMA
Tb Üstün, Chatterji, Rehm, Measuring Health and Disability, Manual for WHO Disability Assessment Schedule
Thoracic, Chinese Association of Chest Physicians Critical Care G
Wan, Wang, Mathur, Chan, Yan et al., Molnupiravir and nirmatrelvir-ritonavir reduce mortality risk during post-acute COVID-19 phase, J Infect
Wang, Yu, Yu, Wang, Chen et al., Clinical features and outcomes of hospitalized patients with COVID-19 during the Omicron wave in Shanghai, China. J Infect
Wbc, None
Xu, Fan, Wang, Zou, Yu et al., Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect
Ye, Wang, Mao, The pathogenesis and treatment of the ;Cytokine Storm' in COVID-19, J Infect
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation (Camb)
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
DOI record:
{
"DOI": "10.1101/2023.05.10.23289325",
"URL": "http://dx.doi.org/10.1101/2023.05.10.23289325",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>The sixty-day effects of initial composite interventions for the treatment of severely and critically ill patients with COVID-19 are not fully assessed.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Using a bayesian piecewise exponential model, we analyzed the 60-day mortality, health-related quality of life (HRQoL) and disability in 1082 severely and critically patients with COVID-19 between December 8, 2022 and February 9, 2023 in Shanghai, China. The final 60-day follow-up was completed on April 10, 2023.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 1082 patients (mean age, 78.0 years), 421 [38.9%] women), 139 patients (12.9%) died within 60 days. Azvudine had a 99.8% probability of improving 2-month survival (adjusted HR, 0.44 [95% credible interval, 0.24-0.79]) and Paxlovid had a 91.9% probability of improving 2-month survival (adjusted HR, 0.71 [95% credible interval, 0.44-1.14]) compared with the control. IL-6 receptor antagonist, Baricitinib, and a-thymosin each had a high probability of benefit (99.5%, 99.4%, and 97.5%, respectively) compared to their controls, while the probability of trail-defined statistical futility (HR >0.83) was high for therapeutic anticoagulation (99.8%; HR, 1.64 [95% CrI, 1.06-2.50]), and glucocorticoid (91.4%; HR, 1.20 [95% CrI, 0.71-2.16]). Paxlovid, Azvudine and therapeutic anticoagulation showed significant reduction in disability (p<0.05)</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Among severely and critically ill patients with COVID-19 who received 1 or more therapeutic interventions, treatment with Azvudine had a high probability of improved 60-day mortality compared with the control, indicating its potential in resource-limited scenario. Treatment with IL-6 receptor antagonist, Baricitinib, and a-thymosin also had high probabilities of benefit of improving 2-month survival, among which a-thymosin could improve HRQoL. Treatment with Paxlovid, Azvudine and therapeutic anticoagulation could significantly reduce disability at day 60.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2023,
5,
18
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6345-5350",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shao",
"given": "Jiasheng",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0814-0548",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fan",
"given": "Rong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2275-3575",
"affiliation": [],
"authenticated-orcid": false,
"family": "Guo",
"given": "Chengnan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Xuyuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Runsheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Fengdi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Jianrong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Gang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Liou",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
5,
15
]
],
"date-time": "2023-05-15T15:53:26Z",
"timestamp": 1684166006000
},
"deposited": {
"date-parts": [
[
2023,
5,
20
]
],
"date-time": "2023-05-20T12:35:26Z",
"timestamp": 1684586126000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T19:45:14Z",
"timestamp": 1708717514182
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2023,
5,
14
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.05.10.23289325",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
5,
14
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
5,
14
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1101/2022.12.29.22284048",
"doi-asserted-by": "crossref",
"key": "2023052005351203000_2023.05.10.23289325v3.1",
"unstructured": "Ioannidis JPA , Zonta F , Levitt M . Estimates of COVID-19 deaths in Mainland China after abandoning zero COVID policy. Eur J Clin Invest. 2023:e13956."
},
{
"DOI": "10.1136/bmj.p2",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.2"
},
{
"DOI": "10.1016/j.jinf.2022.08.001",
"article-title": "Clinical features and outcomes of hospitalized patients with COVID-19 during the Omicron wave in Shanghai, China",
"doi-asserted-by": "crossref",
"first-page": "e27",
"issue": "1",
"journal-title": "J Infect",
"key": "2023052005351203000_2023.05.10.23289325v3.3",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.3390/vaccines10091409",
"doi-asserted-by": "crossref",
"key": "2023052005351203000_2023.05.10.23289325v3.4",
"unstructured": "Shao J , Fan R , Hu J , Zhang T , Lee C , Huang X , et al. Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China. Vaccines (Basel). 2022;10(9)."
},
{
"DOI": "10.1016/S0140-6736(21)01755-4/ATTACHMENT/5440AE6D-9DED-4100-8EE6-77F72BA95154/MMC1.PDF",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.5"
},
{
"DOI": "10.1016/S2213-2600(22)00126-6",
"article-title": "Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study",
"doi-asserted-by": "crossref",
"first-page": "863",
"issue": "9",
"journal-title": "Lancet Respir Med",
"key": "2023052005351203000_2023.05.10.23289325v3.6",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2020.03.03",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.7"
},
{
"DOI": "10.1016/j.jinf.2022.12.027",
"article-title": "Remdesivir and survival outcomes in critically ill patients with COVID-19: A multicentre observational cohort study",
"doi-asserted-by": "crossref",
"first-page": "256",
"issue": "3",
"journal-title": "J Infect",
"key": "2023052005351203000_2023.05.10.23289325v3.8",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.23257",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.9"
},
{
"DOI": "10.1001/jama.2021.18178",
"article-title": "Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial",
"author": "Writing Committee for the R-CAPI",
"doi-asserted-by": "crossref",
"first-page": "1690",
"issue": "17",
"journal-title": "JAMA",
"key": "2023052005351203000_2023.05.10.23289325v3.10",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103417",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.11"
},
{
"DOI": "10.1001/jama.2022.2910",
"article-title": "Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial",
"author": "Investigators R-CWCftR-C",
"doi-asserted-by": "crossref",
"first-page": "1247",
"issue": "13",
"journal-title": "JAMA",
"key": "2023052005351203000_2023.05.10.23289325v3.12",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.13"
},
{
"DOI": "10.1016/j.jinf.2023.02.029",
"doi-asserted-by": "crossref",
"key": "2023052005351203000_2023.05.10.23289325v3.14",
"unstructured": "Wan EYF , Wang B , Mathur S , Chan CIY , Yan VKC , Lai FTT , et al. Molnupiravir and nirmatrelvir-ritonavir reduce mortality risk during post-acute COVID-19 phase. J Infect. 2023."
},
{
"key": "2023052005351203000_2023.05.10.23289325v3.15",
"unstructured": "National Health Commission of the People’s Republic of China. Diagnosis and treatment plan for COVID-19 (trial version 10). in Chinese. 2022."
},
{
"DOI": "10.1016/S2213-2600(22)00006-6",
"article-title": "Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial",
"doi-asserted-by": "crossref",
"first-page": "327",
"issue": "4",
"journal-title": "Lancet Respir Med",
"key": "2023052005351203000_2023.05.10.23289325v3.16",
"volume": "10",
"year": "2022"
},
{
"key": "2023052005351203000_2023.05.10.23289325v3.17",
"unstructured": "TB Üstün NK , S Chatterji , J Rehm . Measuring Health and Disability, Manual for WHO Disability Assessment Schedule, WHODAS 2.0. 2010."
},
{
"DOI": "10.1016/j.jval.2016.11.016",
"article-title": "Estimating an EQ-5D-5L Value Set for China",
"doi-asserted-by": "crossref",
"first-page": "662",
"issue": "4",
"journal-title": "Value Health",
"key": "2023052005351203000_2023.05.10.23289325v3.18",
"volume": "20",
"year": "2017"
},
{
"DOI": "10.1080/22221751.2021.2011619",
"article-title": "Outcomes of patients with severe and critical COVID-19 treated with dexamethasone: a prospective cohort study",
"doi-asserted-by": "crossref",
"first-page": "50",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "2023052005351203000_2023.05.10.23289325v3.19",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1186/s13643-021-01838-8",
"article-title": "Patient-important outcomes reported in randomized controlled trials of pharmacologic treatments for COVID-19: a protocol of a META-epidemiological study",
"doi-asserted-by": "crossref",
"first-page": "289",
"issue": "1",
"journal-title": "Syst Rev",
"key": "2023052005351203000_2023.05.10.23289325v3.20",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.lanwpc.2023.100694",
"doi-asserted-by": "crossref",
"key": "2023052005351203000_2023.05.10.23289325v3.21",
"unstructured": "Liu J , Pan X , Zhang S , Li M , Ma K , Fan C , et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Lancet Reg Health West Pac. 2023:100694."
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"doi-asserted-by": "crossref",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "2023052005351203000_2023.05.10.23289325v3.22",
"volume": "6",
"year": "2021"
},
{
"article-title": "Chinese Association of Chest Physicians Critical Care G. [Expert consensus on treatment of severe COVID-19 caused by Omicron variants]",
"first-page": "101",
"issue": "2",
"journal-title": "Zhonghua Jie He He Hu Xi Za Zhi",
"key": "2023052005351203000_2023.05.10.23289325v3.23",
"volume": "46",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2020.04.012",
"doi-asserted-by": "publisher",
"key": "2023052005351203000_2023.05.10.23289325v3.24"
},
{
"DOI": "10.1093/cid/ciaa630",
"article-title": "Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells",
"doi-asserted-by": "crossref",
"first-page": "2150",
"issue": "16",
"journal-title": "Clin Infect Dis",
"key": "2023052005351203000_2023.05.10.23289325v3.25",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2023.109949",
"article-title": "COVID-19 and beyond: Reassessing the role of thymosin alpha1 in lung infections",
"doi-asserted-by": "crossref",
"first-page": "109949",
"journal-title": "Int Immunopharmacol",
"key": "2023052005351203000_2023.05.10.23289325v3.26",
"volume": "117",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2023.1116738",
"article-title": "Benefits of high-dose intravenous immunoglobulin on mortality in patients with severe COVID-19: An updated systematic review and meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "1116738",
"journal-title": "Front Immunol",
"key": "2023052005351203000_2023.05.10.23289325v3.27",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1186/s40001-022-00637-8",
"article-title": "The outcome of using intravenous immunoglobulin (IVIG) in critically ill COVID-19 patients’: a retrospective, multi-centric cohort study",
"doi-asserted-by": "crossref",
"first-page": "18",
"issue": "1",
"journal-title": "Eur J Med Res",
"key": "2023052005351203000_2023.05.10.23289325v3.28",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.23866",
"article-title": "Antiplatelet Therapy in Patients With COVID-19-More Is Less?",
"doi-asserted-by": "crossref",
"first-page": "223",
"issue": "3",
"journal-title": "JAMA",
"key": "2023052005351203000_2023.05.10.23289325v3.29",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.3390/jcm12052038",
"doi-asserted-by": "crossref",
"key": "2023052005351203000_2023.05.10.23289325v3.30",
"unstructured": "Bolek T , Samos M , Jurica J , Stanciakova L , Pec MJ , Skornova I , et al. COVID-19 and the Response to Antiplatelet Therapy. J Clin Med. 2023;12(5)."
},
{
"article-title": "The first Chinese oral anti-COVID-19 drug Azvudine launched",
"first-page": "100321",
"issue": "6",
"journal-title": "Innovation (Camb)",
"key": "2023052005351203000_2023.05.10.23289325v3.31",
"volume": "3",
"year": "2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.05.10.23289325"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China",
"type": "posted-content"
}