Assessment of outcomes following implementation of antiviral treatment guidelines for COVID-19 during the first wave in Thailand
et al., Southeast Asian Journal of Tropical Medicine and Public Health, 52:4, Sep 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
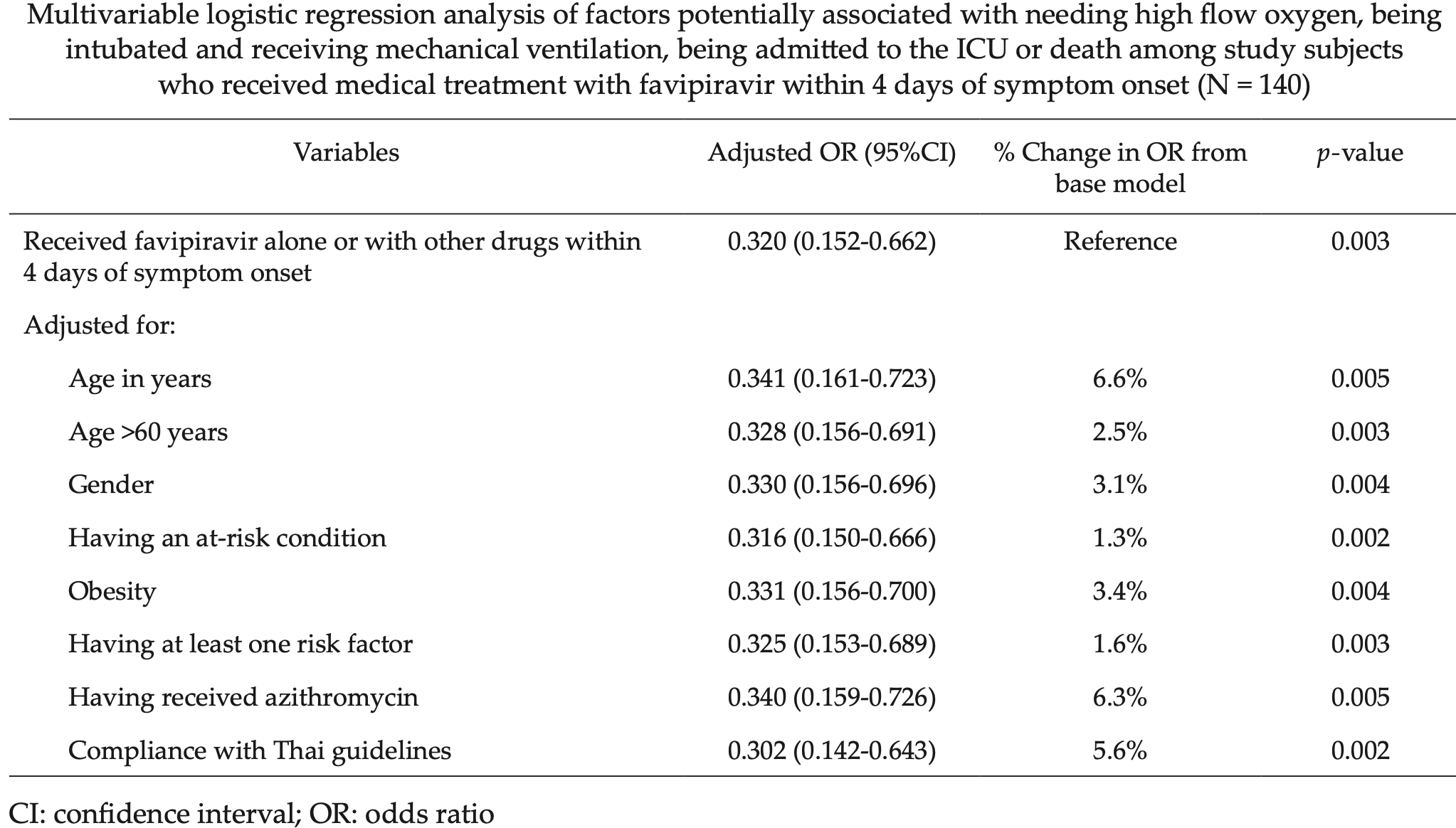
Retrospective 744 hospitalized patients in Thailand, showing lower risk of a poor outcome for favipiravir treatment within 4 days of symptom onset. Early treatment with CQ/HCQ and lopinavir/ritonavir or darunavir/ritonavir also showed lower risk, but without statistical significance. Sample sizes for the number of patients treated within 4 days of symptom onset are not provided.
Study covers favipiravir and HCQ.
|
risk of death, ICU, intubation, or high-flow oxygen, 42.0% lower, OR 0.58, p = 0.37, within 4 days of symptom onset, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sawanpanyalert et al., 9 Sep 2021, retrospective, Thailand, peer-reviewed, 11 authors, dosage varies, this trial uses multiple treatments in the treatment arm (combined with lopinavir/ritonavir or darunavir/ritonavir) - results of individual treatments may vary.
ASSESSEMENT OF OUTCOMES FOLLOWING IMPLEMENTATION OF ANTIVIRAL TREATMENT GUIDELINES FOR COVID-19 DURING THE FIRST WAVE IN THAILAND
Thailand encountered its first coronavirus disease 2019 outbreak in March 2020 and the Thailand Ministry of Public Health rapidly developed COVID-19 treatment guidelines. In this study we aimed to describe the outcomes among patients treated following those initial guidelines and determine factors significantly associated with poor outcomes in order to inform efforts to improve COVID-19 treatment guidelines for Thailand. Nine hospitals in Bangkok submitted data from their COVID-19 patients using standardized case record forms. A poor outcome was defined as death, ICU admission, requiring intubation or requiring high-flow oxygen. Factors associated with these outcomes were assessed. A total of 744 patients (48.8% male) were included in the study. The median (interquartile range) age of study subjects was 37 (27-48) years; 8.4% were aged >60 years, 5.6% of subjects were obese and 16.5% had underlying conditions: obesity, immunocompromised status, diabetes, chronic conditions of lungs, kidneys, liver, cardiovascular or cerebrovascular systems or had an absolute lymphocyte count <1,000 cells/mm 3 . Among symptomatic patients, factors significantly independently associated with a poor outcome were: age >60 years (adjusted odds ratio (
References
Cai, Yanga, Liu, Experimental t r e a t m e n t w i t h f a v i p i r a v i r f o r COVID-19: an open-label control study, Engineering
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19, N Engl J Med
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without azithromycin in mild-tomoderate COVID-19, N Engl J Med
Chen, Zhang, Huang, Favipiravir versus arbidol for COVID-19: a randomized clinical trial, doi:10.1101/2020.03.17.20037432v4.full.pdf+html
Chu, Cheng, Hung, Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings, Thorax
Doi, Hibino, Hase, A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrob Agents Chemother
Fujifilm Toyama, Co L T D, AV I G A N Ta b l e t s 2 0 0 m g : Prescribing information
Hassanipour, Arab-Zozani, Amani, H E I D A R Z A D F , F A T H A L I P O U R, Martinez-De-Hoyo, The efficacy and safety of favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep
Hu, Wang, The clinical characteristics and risk factors of severe COVID-19, Gerontology
Ivashchenko, Dmitriev, Vostokova, AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a Phase II/III multicenter randomized clinical trial, Clin Infect Dis
Liu, Cao, Xu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Manosuthi, Jeungsmarn, Okada, Nasopharyngeal SARS-CoV-2 viral load response among COVID-19 patients receiving favipiravir, N Engl J Med
Recovery Collaborative, Lopinavir-ritonavir in patients admitted to hospital with COVID-19 ( R E C O V E R Y ) : a r a n d o m i s e d , controlled, open-label, platform trial, N Engl J Med
Rosenberg, Dufort, Udo, A s s o c i a t i o n o f t r e a t m e n t w i t h hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State, JAMA
Sim, Chidambaram, Wong, Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study, Lancet Reg Health West Pac
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Weiss, Jellingsø, Sommera, Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: a systematic review and meta-analysis, EBioMedicine
Wolff, Nee, Hickey, Marschollek, Risk factors for COVID-19 severity and fatality: a structured literature review, Infection
