Safety and efficacy of COROPROTECT kit as an add-on therapy in the management of mild-to-moderate COVID-19: A randomized, placebo-controlled trial
et al., AYU (An International Quarterly Journal of Research in Ayurveda), doi:10.4103/ayu.ayu_92_22, CTRI/2021/08/036010, Feb 2023
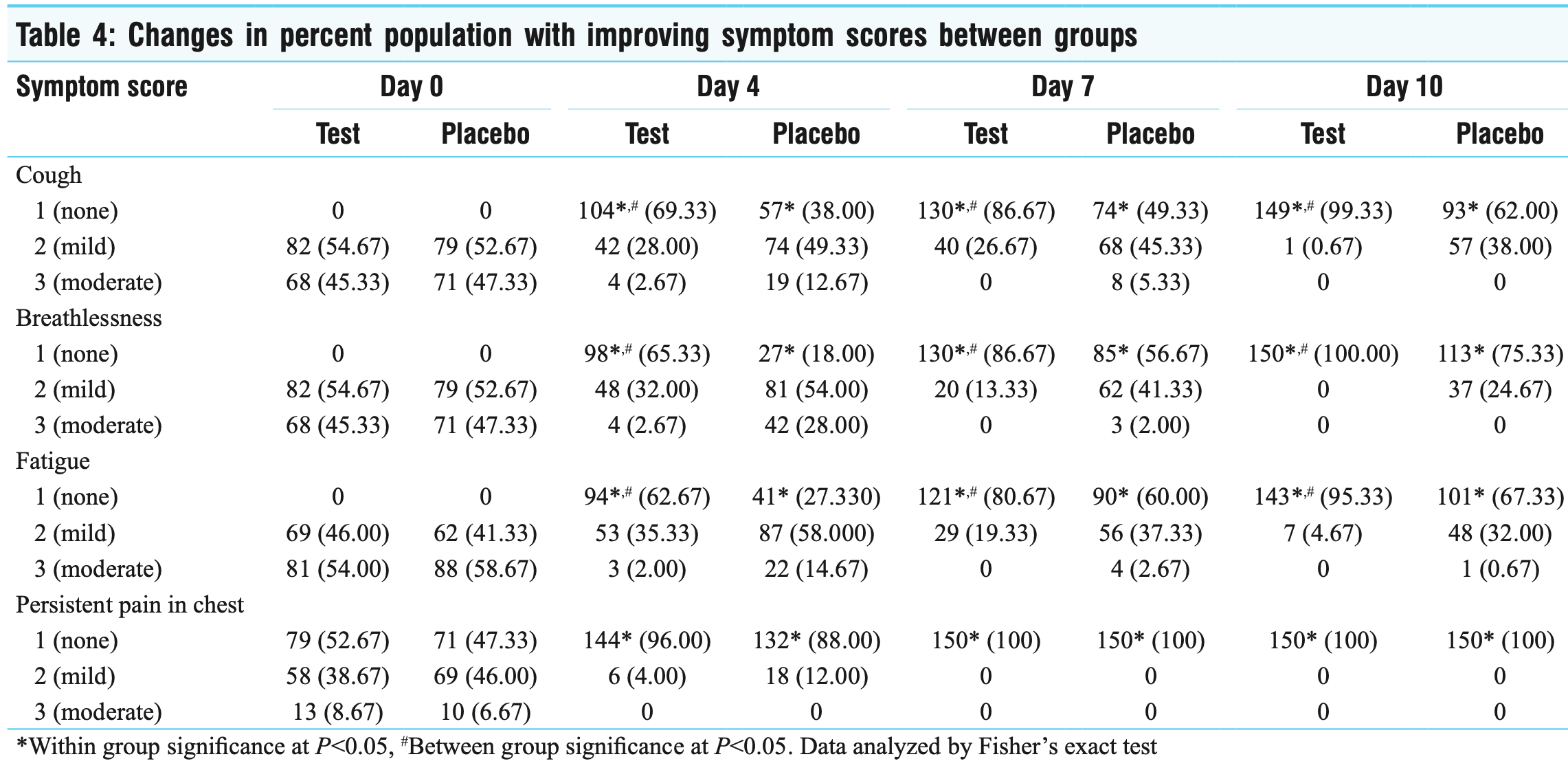
RCT with 300 mild to moderate hospitalized COVID-19 patients, showing faster recovery, faster viral clearance, and a reduction in inflammatory markers with COROPROTECT, which includes curcumin, andrographis, and several additional treatments with preclinical evidence supporting efficacy. Symptoms resolved much faster with treatment, for example at day 4 only 3% of treatment patients reported moderate dyspnea, compared to 28% for placebo. The treatment group had a significantly higher percentage of patients testing negative on days 4, 7, and 10 compared to placebo. The treatment group also showed greater reductions in CRP, LDH, and IL-6 levels. No adverse events were reported. There was no progression to serious outcomes in either group.
This study is excluded in meta-analysis:
many combined treatments which may significantly contribute to the effect seen.
Study covers curcumin and andrographolide.
|
risk of no recovery, 86.2% lower, RR 0.14, p < 0.001, treatment 150, control 150, combined symptoms.
|
|
risk of no recovery, 90.5% lower, RR 0.10, p < 0.001, treatment 4 of 150 (2.7%), control 42 of 150 (28.0%), NNT 3.9, mid-recovery, moderate symptoms, day 4, dyspnea.
|
|
risk of no recovery, 86.4% lower, RR 0.14, p < 0.001, treatment 3 of 150 (2.0%), control 22 of 150 (14.7%), NNT 7.9, mid-recovery, moderate symptoms, day 4, fatigue.
|
|
risk of no recovery, 78.9% lower, RR 0.21, p = 0.002, treatment 4 of 150 (2.7%), control 19 of 150 (12.7%), NNT 10.0, mid-recovery, moderate symptoms, day 4, cough.
|
|
risk of no recovery, 90.3% lower, RR 0.10, p = 0.006, treatment 150, control 150, combined symptoms.
|
|
risk of no recovery, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 150 (0.0%), control 3 of 150 (2.0%), NNT 50, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), moderate symptoms, day 7, dyspnea.
|
|
risk of no recovery, 88.9% lower, RR 0.11, p = 0.12, treatment 0 of 150 (0.0%), control 4 of 150 (2.7%), NNT 38, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), moderate symptoms, day 7, fatigue.
|
|
risk of no recovery, 94.1% lower, RR 0.06, p = 0.007, treatment 0 of 150 (0.0%), control 8 of 150 (5.3%), NNT 19, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), moderate symptoms, day 7, cough.
|
|
risk of no viral clearance, 53.2% lower, RR 0.47, p < 0.001, treatment 44 of 150 (29.3%), control 94 of 150 (62.7%), NNT 3.0, mid-recovery, day 4.
|
|
risk of no viral clearance, 95.0% lower, RR 0.05, p < 0.001, treatment 1 of 150 (0.7%), control 20 of 150 (13.3%), NNT 7.9, day 7.
|
|
risk of no viral clearance, 95.2% lower, RR 0.05, p = 0.002, treatment 0 of 150 (0.0%), control 10 of 150 (6.7%), NNT 15, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 10.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Savaliya et al., 21 Feb 2023, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, mean age 35.8, 4 authors, this trial uses multiple treatments in the treatment arm (combined with combined treatments) - results of individual treatments may vary, trial CTRI/2021/08/036010.
Contact: dheeraj@mprex.in.
Safety and efficacy of COROPROTECT kit as an add-on therapy in the management of mild-to-moderate COVID-19: A randomized, placebo-controlled trial
AYU (An International Quarterly Journal of Research in Ayurveda), doi:10.4103/ayu.ayu_92_22
Background: The constructive role of Ayurveda in managing COVID-19 has been widely discussed, with identified herbs showing immunomodulatory and anti-viral potential. However, clinical trials examining their safety and efficacy are limited. Aim: The aim of this study is to determine the efficacy of COROPROTECT kit, a proprietary Ayurvedic formulation, in COVID-19. Materials and method: Randomized, placebo-controlled trial with 312 mild to moderate hospitalized COVID-19 patients. Groups received COROPROTECT or placebo for 10 days alongside standard care. Results: The outcome measures included the number of days taken to reverse the reverse transcriptase-polymerase chain reaction (RT-PCR) status, reduction in symptoms and inflammatory markers. Fisher exact test was used to analyze the changes between categorical variables, whereas the comparative effect of therapy in both groups on inflammatory markers and safety biochemical parameters was analyzed using Student's t test. A total of 300 patients completed the study without any adverse events. The COROPROTECT kit group exhibited a statistically significant higher percentage of patients testing negative on days 4, 7, and 10 compared to the placebo group. A within group analysis showed that trial group to significantly reduced the levels of C-reactive protein (P = 0.03), lactate dehydrogenase (P < 0.001), and interleukin-6 (P = 0.01). Subjects of the trial group experienced complete relief from cough (69.33%), breathlessness (65.33%), and fatigue (62.67%) within 4 days. In contrast, the placebo group had 20%-40% of participants with mild symptoms persisting until day 10.
Conclusion: This study suggests potential future implications, indicating a faster RT-PCR negativity, reduced COVID-19 severity, and inflammatory markers, along with early symptomatic recovery. The COROPROTECT kit proved safe, facilitating an accelerated clinical recovery compared to conventional care.
Conflicts of interest There are no conflicts of interest.
Supplementary File About the trial drug Gplife "COROPROTECT tablet and COROPROTECT dry syrup" are successfully tested and demonstrated good efficacy in Anti-SARS-CoV-2 activity with 85% and 72% SARS-CoV-2 inhibition in 24 hrs was carried out at Government of India approved, Department of Biotechnology, Institute of Life science-Bhuvneshwar. Both these products have received Ayurvedic medicine license by FDCA, Gujarat. The COROPROTECT tablet and COROPROTECT dry syrup formulations were tested for its antioxidant activity by In vitro analysis, and standardization of the both was carried out by using HPLC method.
Detailed description of the ingredients, quantity and specifications of preparation of COROPROTECT kit
Each coated tablet contains
References
Adluri, Tripathi, Understanding COVID-19 pandemic -A comprehensive Ayurvedic perspective, J Ayurveda Integr Med
Ali, Ayush -64" -A new anti malarial herbal compound, Indian J Pathol Microbiol
Baker, Hanrath, Schim Van Der Loeff, Kay, Back et al., National early warning score 2 (NEWS2) to identify inpatient COVID-19 deterioration: A retrospective analysis, Clin Med (Lond)
Balkrishna, Khandrika, Varshney, Giloy Ghanvati (Tinospora cordifolia (Willd.) Hook. F. and Thomson) reversed SARS-CoV-2 viral spike-protein induced disease phenotype in the xenotransplant model of humanized zebrafish, Front Pharmacol
Borse, Joshi, Saggam, Bhat, Walia et al., Ayurveda botanicals in COVID-19 management: An in silico multitarget approach, PLoS One
Devpura, Tomar, Nathiya, Sharma, Bhandari et al., Randomized placebo-controlled pilot clinical trial on the efficacy of Ayurvedic treatment regime on COVID-19 positive patients, Phytomedicine
Diomede, Beeg, Gamba, Fumagalli, Gobbi et al., Can antiviral activity of licorice help fight COVID-19 infection?, Biomolecules
Garcia, Immune response, inflammation, and the clinical spectrum of COVID-19, Front Immunol
Henry, Aggarwal, Wong, Benoit, Vikse et al., Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis, Am J Emerg Med
Kataria, Sharma, Ram, Deswal, Singh et al., A pilot clinical study of an add-on Ayurvedic formulation containing Tinospora cordifolia and Piper longum in mild to moderate COVID-19, J Ayurveda Integr Med
Liu, Yang, Zhang, Huang, Wang et al., Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury, Sci China Life Sci
Patwardhan, Chavan-Gautam, Gautam, Sci, Ayurveda Rasayana in Prophylaxis of COVID-19
Payyappallimana, Patwardhan, Mangalath, Kessler, Jayasundar et al., The COVID-19 pandemic and the relevance of Ayurveda's whole systems approach to health and disease management, J Altern Complement Med
Reddy, Gosavi, Yadav, Rai, Holay et al., AYUSH-64 as an add-on to standard care in asymptomatic and mild cases of COVID-19: A randomized controlled trial, Ayu
Saggam, Limgaokar, Borse, Chavan-Gautam, Dixit et al., somnifera (L.) Dunal: Opportunity for clinical repurposing in COVID-19 management, Front Pharmacol
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal, J Heart Lung Transplant
Singh, Goel, Bourgeade, Aleya, Tewari, Ayurveda Rasayana as antivirals and immunomodulators: Potential applications in COVID-19, Environ Sci Pollut Res Int
Singh, Srivastava, Yadav, Rai, Jameela et al., AYUSH-64 as an adjunct to standard care in mild to moderate COVID-19: An open-label randomized controlled trial in Chandigarh, India, Complement Ther Med
Sulaiman, Deepak, Ramesh, Mahesh, Anandan et al., Chemical profiling of selected Ayurveda formulations recommended for COVID-19, Beni Suef Univ J Basic Appl Sci
Thakar, Panara, Patel, Bhagiya, Goyal et al., Add-on Ayurveda treatment for early stage COVID-19: A single center retrospective cohort study from Gujarat, India, J Evid Based Integr Med, doi:10.1177/2515690X211020685
Vargas-Vargas, Cortés-Rojo, Ferritin levels and COVID-19, Rev Panam Salud Publica
DOI record:
{
"DOI": "10.4103/ayu.ayu_92_22",
"ISSN": [
"0974-8520",
"0976-9382"
],
"URL": "http://dx.doi.org/10.4103/ayu.ayu_92_22",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background:</jats:title>\n <jats:p>The constructive role of Ayurveda in managing COVID-19 has been widely discussed, with identified herbs showing immunomodulatory and anti-viral potential. However, clinical trials examining their safety and efficacy are limited.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Aim:</jats:title>\n <jats:p>The aim of this study is to determine the efficacy of COROPROTECT kit, a proprietary Ayurvedic formulation, in COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Materials and method:</jats:title>\n <jats:p>Randomized, placebo-controlled trial with 312 mild to moderate hospitalized COVID-19 patients. Groups received COROPROTECT or placebo for 10 days alongside standard care.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results:</jats:title>\n <jats:p>The outcome measures included the number of days taken to reverse the reverse transcriptase-polymerase chain reaction (RT-PCR) status, reduction in symptoms and inflammatory markers. Fisher exact test was used to analyze the changes between categorical variables, whereas the comparative effect of therapy in both groups on inflammatory markers and safety biochemical parameters was analyzed using Student’s <jats:italic toggle=\"yes\">t</jats:italic> test. A total of 300 patients completed the study without any adverse events. The COROPROTECT kit group exhibited a statistically significant higher percentage of patients testing negative on days 4, 7, and 10 compared to the placebo group. A within group analysis showed that trial group to significantly reduced the levels of C-reactive protein (<jats:italic toggle=\"yes\">P</jats:italic> = 0.03), lactate dehydrogenase (<jats:italic toggle=\"yes\">P</jats:italic> < 0.001), and interleukin-6 (<jats:italic toggle=\"yes\">P</jats:italic> = 0.01). Subjects of the trial group experienced complete relief from cough (69.33%), breathlessness (65.33%), and fatigue (62.67%) within 4 days. In contrast, the placebo group had 20%–40% of participants with mild symptoms persisting until day 10.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion:</jats:title>\n <jats:p>This study suggests potential future implications, indicating a faster RT-PCR negativity, reduced COVID-19 severity, and inflammatory markers, along with early symptomatic recovery. The COROPROTECT kit proved safe, facilitating an accelerated clinical recovery compared to conventional care.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Research and Development Department, Gplife Healthcare Pvt., Ltd., Surat, Gujarat, India"
}
],
"family": "Savaliya",
"given": "Chetan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Research and Development Department, Gplife Healthcare Pvt., Ltd., Surat, Gujarat, India"
}
],
"family": "Pandya",
"given": "Shridhar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research and Development Department, Gplife Healthcare Pvt., Ltd., Surat, Gujarat, India"
}
],
"family": "Thumar",
"given": "Kamalesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Director, Mprex Healthcare Pvt., Ltd., Pune, Maharashtra, India"
}
],
"family": "Nagore",
"given": "Dheeraj",
"sequence": "additional"
}
],
"container-title": "AYU (An International Quarterly Journal of Research in Ayurveda)",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T20:00:10Z",
"timestamp": 1708718410000
},
"deposited": {
"date-parts": [
[
2024,
2,
27
]
],
"date-time": "2024-02-27T23:01:24Z",
"timestamp": 1709074884000
},
"indexed": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T00:44:07Z",
"timestamp": 1709081047799
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.4103/ayu.ayu_92_22",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2581",
"original-title": [],
"page": "9-16",
"prefix": "10.4103",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
21
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Medknow",
"reference": [
{
"DOI": "10.4103/ayu.ayu_14_21",
"article-title": "AYUSH-64 as an add-on to standard care in asymptomatic and mild cases of COVID-19: A randomized controlled trial",
"author": "Reddy",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Ayu",
"key": "R2-20240227",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1016/j.jaim.2020.08.001",
"article-title": "Understanding COVID-19 pandemic –A comprehensive Ayurvedic perspective",
"author": "Adluri",
"doi-asserted-by": "crossref",
"first-page": "100348",
"journal-title": "J Ayurveda Integr Med",
"key": "R3-20240227",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0248479",
"article-title": "Ayurveda botanicals in COVID-19 management: An in silico multi-target approach",
"author": "Borse",
"doi-asserted-by": "crossref",
"first-page": "e0248479",
"journal-title": "PLoS One",
"key": "R4-20240227",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1007/s11356-021-16280-5",
"article-title": "Ayurveda Rasayana as antivirals and immunomodulators: Potential applications in COVID-19",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "55925",
"journal-title": "Environ Sci Pollut Res Int",
"key": "R6-20240227",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.phymed.2021.153494",
"article-title": "Randomized placebo-controlled pilot clinical trial on the efficacy of Ayurvedic treatment regime on COVID-19 positive patients",
"author": "Devpura",
"doi-asserted-by": "crossref",
"first-page": "153494",
"journal-title": "Phytomedicine",
"key": "R7-20240227",
"volume": "84",
"year": "2021"
},
{
"article-title": "Add-on Ayurveda treatment for early stage COVID-19: A single center retrospective cohort study from Gujarat, India",
"author": "Thakar",
"first-page": "26",
"journal-title": "J Evid Based Integr Med",
"key": "R8-20240227",
"year": "2021"
},
{
"DOI": "10.1186/s43088-020-00089-1",
"article-title": "Chemical profiling of selected Ayurveda formulations recommended for COVID-19",
"author": "Sulaiman",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Beni Suef Univ J Basic Appl Sci",
"key": "R9-20240227",
"volume": "10",
"year": "2021"
},
{
"article-title": "“Ayush -64”–A new anti malarial herbal compound",
"author": "Ali",
"first-page": "499",
"journal-title": "Indian J Pathol Microbiol",
"key": "R10-20240227",
"volume": "39",
"year": "1996"
},
{
"DOI": "10.1089/acm.2020.0370",
"article-title": "The COVID-19 pandemic and the relevance of Ayurveda's whole systems approach to health and disease management",
"author": "Payyappallimana",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "J Altern Complement Med",
"key": "R11-20240227",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"article-title": "COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal",
"author": "Siddiqi",
"doi-asserted-by": "crossref",
"first-page": "405",
"journal-title": "J Heart Lung Transplant",
"key": "R12-20240227",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.7861/clinmed.2020-0688",
"article-title": "National early warning score 2 (NEWS2) to identify inpatient COVID-19 deterioration: A retrospective analysis",
"author": "Baker",
"doi-asserted-by": "crossref",
"first-page": "84",
"journal-title": "Clin Med (Lond)",
"key": "R15-20240227",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.jaim.2021.05.008",
"article-title": "Apilot clinical study of an add-on Ayurvedic formulation containing Tinospora cordifolia and Piper longum in mild to moderate COVID-19",
"author": "Kataria",
"doi-asserted-by": "crossref",
"first-page": "100454",
"journal-title": "J Ayurveda Integr Med",
"key": "R17-20240227",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.623795",
"article-title": "Withania somnifera (L.). Dunal: Opportunity for clinical repurposing in COVID-19 management",
"author": "Saggam",
"doi-asserted-by": "crossref",
"first-page": "623795",
"journal-title": "Front Pharmacol",
"key": "R18-20240227",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/biom11060855",
"article-title": "Can antiviral activity of licorice help fight COVID-19 infection?",
"author": "Diomede",
"doi-asserted-by": "crossref",
"first-page": "855",
"journal-title": "Biomolecules",
"key": "R19-20240227",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.635510",
"article-title": "Giloy Ghanvati (Tinospora cordifolia (Willd.). Hook. F. and Thomson) reversed SARS-CoV-2 viral spike-protein induced disease phenotype in the xenotransplant model of humanized zebrafish",
"author": "Balkrishna",
"doi-asserted-by": "crossref",
"first-page": "635510",
"journal-title": "Front Pharmacol",
"key": "R20-20240227",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.01441",
"article-title": "Immune response, inflammation, and the clinical spectrum of COVID-19",
"author": "Garcia",
"doi-asserted-by": "crossref",
"first-page": "1441",
"journal-title": "Front Immunol",
"key": "R21-20240227",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1007/s11427-020-1643-8",
"article-title": "Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "364",
"journal-title": "Sci China Life Sci",
"key": "R22-20240227",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1016/j.ajem.2020.05.073",
"article-title": "Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis",
"author": "Henry",
"doi-asserted-by": "crossref",
"first-page": "1722",
"journal-title": "Am J Emerg Med",
"key": "R23-20240227",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.ctim.2022.102814",
"article-title": "AYUSH-64 as an adjunct to standard care in mild to moderate COVID-19: An open-label randomized controlled trial in Chandigarh, India",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "102814",
"journal-title": "Complement Ther Med",
"key": "R24-20240227",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.26633/RPSP.2020.72",
"article-title": "Ferritin levels and COVID-19",
"author": "Vargas-Vargas",
"doi-asserted-by": "crossref",
"first-page": "e72",
"journal-title": "Rev Panam Salud Publica",
"key": "R25-20240227",
"volume": "44",
"year": "2020"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.4103/ayu.ayu_92_22"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Safety and efficacy of COROPROTECT kit as an add-on therapy in the management of mild-to-moderate COVID-19: A randomized, placebo-controlled trial",
"type": "journal-article",
"volume": "44"
}
savaliya