Oral Melatonin in Critically Ill Patients With COVID‐19: A Quasi‐Experimental Pragmatic Trial
et al., Journal of Medical Virology, doi:10.1002/jmv.70807, Jul 2025
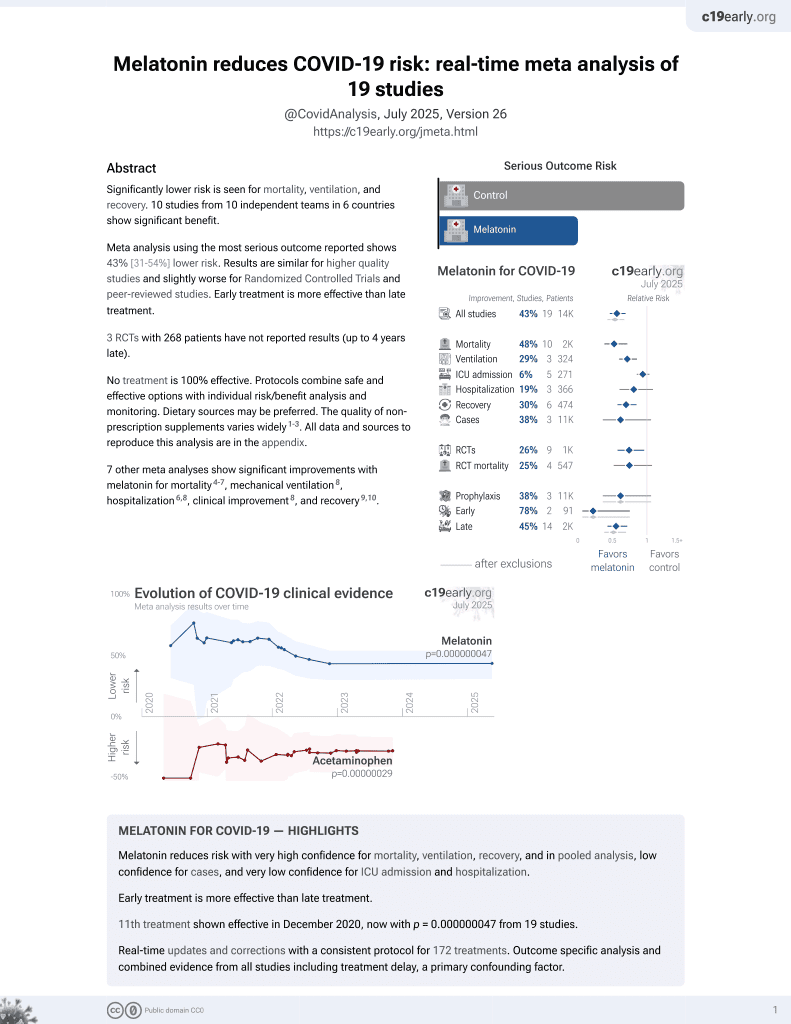
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
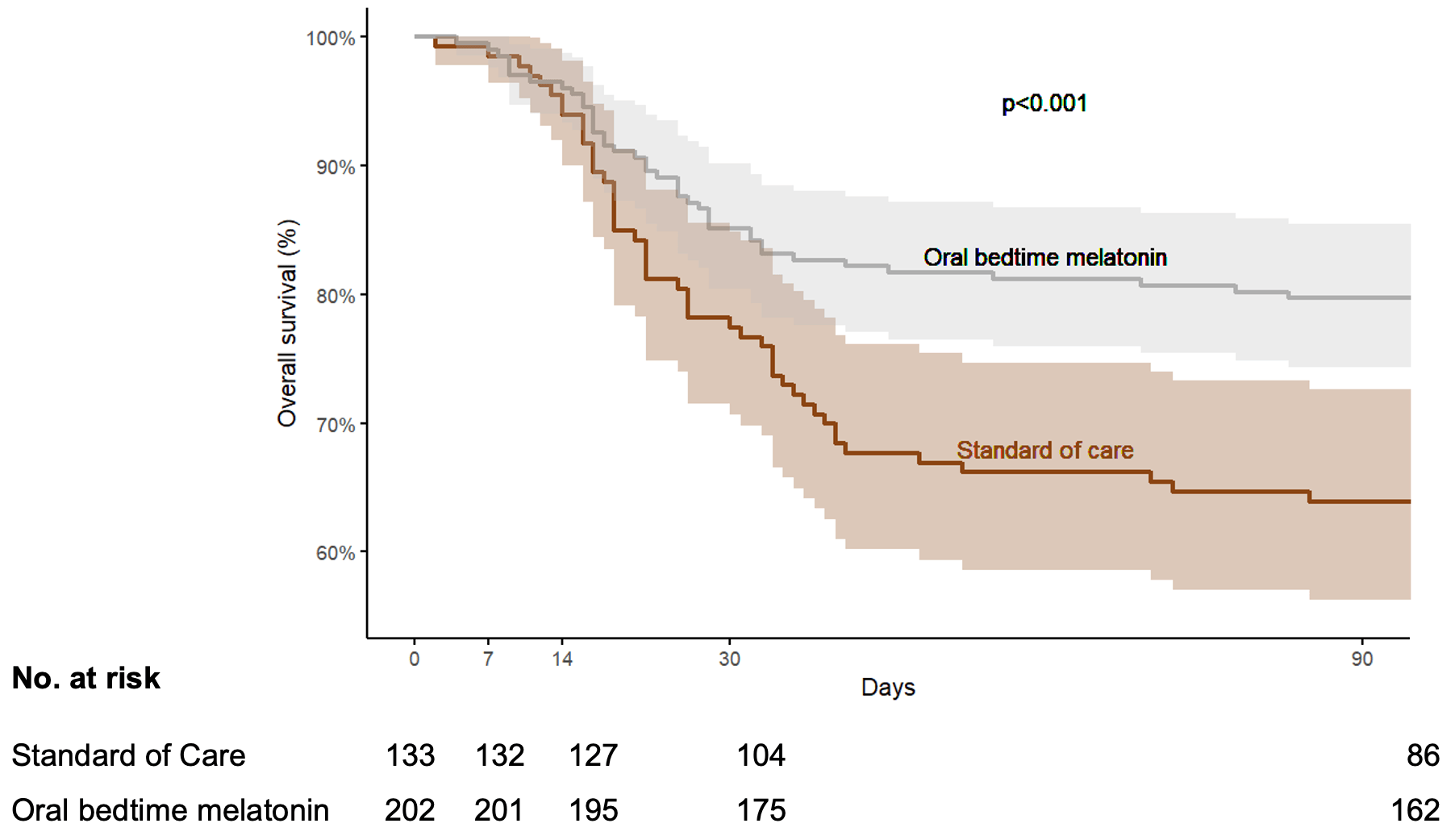
Quasi-experimental study of 335 critically ill COVID-19 patients showing significantly lower 90-day mortality with high-dose oral melatonin (50-200mg daily). The study used alternating control and treatment periods, with 202 patients receiving melatonin plus standard care versus 133 receiving standard care alone. Mortality was reduced from 36.1% to 20.8% (OR 0.46 [0.28-0.76]), with lower organ dysfunction scores and fewer severe adverse events in the melatonin group. The quasi-experimental design introduces potential confounding, though authors attempted to control for temporal changes in treatment protocols and COVID variants.
|
risk of death, 41.9% lower, RR 0.58, p = 0.002, treatment 42 of 202 (20.8%), control 48 of 133 (36.1%), NNT 6.5, adjusted per study, odds ratio converted to relative risk, day 90.
|
|
risk of mechanical ventilation, 54.0% lower, OR 0.46, p = 0.002, treatment 202, control 133, adjusted per study, day 90, RR approximated with OR.
|
|
ICU time, 31.2% lower, relative time 0.69, p = 0.002, treatment mean 11.0 (±68.9) n=202, control mean 16.0 (±76.5) n=133.
|
|
hospitalization time, 21.4% lower, relative time 0.79, p = 0.007, treatment mean 22.0 (±77.0) n=202, control mean 28.0 (±63.3) n=133.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sánchez-García et al., 21 Jul 2025, prospective, Spain, peer-reviewed, 23 authors, study period 30 March, 2020 - 27 April, 2021.
Contact: miguel.sanchez@salud.madrid.org.
Oral Melatonin in Critically Ill Patients With COVID‐19: A Quasi‐Experimental Pragmatic Trial
Journal of Medical Virology, doi:10.1002/jmv.70807
Melatonin has demonstrated antioxidant, anti-inflammatory, and potential antiviral properties. Its therapeutic role in critically ill COVID-19 patients admitted to intensive care was underexplored at the start of the pandemic. We conducted a quasiexperimental, pragmatic study over 4 consecutive uninterrupted time periods alternating control groups receiving standard of care (SoC) with treatment groups receiving SoC plus high-dose oral bedtime melatonin (50-200 mg) (OBM). The primary endpoint was 90-day mortality; secondary outcomes included sequential organ failure assessment (SOFA) scores at 4, 7, 14, and 30 days and pre-defined severe adverse events (SAEs). A total of 335 of 339 consecutive patients with a predicted stay > 48 h were enrolled; 202 received OBM with SoC and 133 received SoC alone. OBM was dispensed during the second (n = 162) and fourth (n = 40) study periods after the first (n = 40) and third (n = 93) control group periods, respectively. Melatonin therapy was associated with significantly lower 90-day mortality (20.8% vs. 36.1%, OR 0.46, 95% CI 0.28-0.76). Subjects receiving melatonin had lower SOFA scores on Day 4 and subsequent study visits. SAEs occurred in 84 (41.6%) subjects on OBM and in 80 (60.2%) receiving SoC (risk ratio 0.68, 95% CI 0.54-0.87; p = 0.001). High-dose oral melatonin was safe and associated with improved clinical outcomes. Further evaluation of melatonin and its potential antiviral effects in future epidemics is warranted.
Author Contributions The structure of the electronic case report form and the study variables were generated and approved by all authors, who participated in the design of the database and reviewed the data. M.S.G. created the first draft of the manuscript. A.N.R. performed the statistical analyses. the Departments of Medicine, Pharmacy and Microbiology at Hospital Clínico San Carlos for their help and cooperation.
Ethics Statement The local Ethics Review Board approved the study (20/352-E_COVID) and granted a waiver for informed consent. (Supporting Information S1: Appendix 1).
Consent All authors had access to the database and reviewed and edited the final version of the manuscript and agreed to publish.
Conflicts of Interest The authors declare no conflicts of interest.
Supporting Information Additional supporting information can be found online in the Supporting Information section. Supplemental Material JMedVirol. Supplemental Figures JMedVirol. Supplemental Tables JMedVirol.
References
Alhazzani, Møller, Arabi, Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults With Coronavirus Disease 2019 (COVID-19), Critical Care Medicine
Ameri, Frouz Asadi, Ziaei, Efficacy and Safety of Oral Melatonin in Patients With Severe COVID-19: A Randomized Controlled Trial, Inflammopharmacology
Andersen, Gögenur, Rosenberg, Reiter, Pharmacokinetics of Melatonin: The Missing Link in Clinical Efficacy?, Clinical Pharmacokinetics
Andersen, Werner, Rosenkilde, Pharmacokinetics of High-Dose Intravenous Melatonin in Humans, Journal of Clinical Pharmacology
Andersen, Werner, Rosenkilde, Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers, BMC Pharmacology and Toxicology
Anderson, Reiter, Melatonin: Roles in Influenza, Covid-19, and Other Viral Infections, Reviews in Medical Virology
Ards Definition, Task, Ranieri, Rubenfeld, Acute Respiratory Distress Syndrome: The Berlin Definition, Journal of the American Medical Association
Aziz, Arabi, Alhazzani, Managing ICU Surge During the COVID-19 Crisis: Rapid Guidelines, Intensive Care Medicine
Ballesteros Sanz, Hernández-Tejedor, Estella, Recomendaciones De «Hacer» Y «No Hacer» En El Tratamiento De Los Pacientes Críticos Ante La Pandemia Por Coronavirus Causante De COVID-19 De Los Grupos De Trabajo De La Sociedad Española De Medicina Intensiva, Crítica, Y Unidades Coronarias (SEMICYUC)
Bhatraju, Ghassemieh, Nichols, Covid-19 in Critically Ill Patients in the Seattle Region-Case Series, New England Journal of Medicine
Carrillo-Vico, Lardone, Naji, Beneficial Pleiotropic Actions of Melatonin in an Experimental Model of Septic Shock in Mice: Regulation of Pro-/Anti-Inflammatory Cytokine Network, Protection Against Oxidative Damage and Anti-Apoptotic Effects, Journal of Pineal Research
Casey, Beskow, Brown, Use of Pragmatic and Explanatory Trial Designs in Acute Care Research: Lessons From Covid-19, Lancet Respiratory Medicine
Chaplin, Cook, Zurovac, The Internal and External Validity of the Regression Discontinuity Design: A Meta-Analysis of 15 Within-Study Comparisons, Journal of Policy Analysis and Management
Chavarría, Vázquez, Cherit, Antioxidants and Pentoxifylline as Coadjuvant Measures to Standard Therapy to Improve Prognosis of Patients With Pneumonia by COVID-19, Computational and Structural Biotechnology Journal
Colunga Biancatelli, Berrill, Mohammed, Marik, Melatonin for the Treatment of Sepsis: The Scientific Rationale, Journal of Thoracic Disease
Ernst, Canter, Limitations of 'Pragmatic' Trials, Postgraduate Medical Journal
Ford, Norrie, Pragmatic Trials, New England Journal of Medicine
Galley, Allen, Colin, Galt, Webster, Dose Assessment of Melatonin in Sepsis (DAMSEL2) Study: Pharmacokinetics of Two Doses of Oral Melatonin in Patients With Sepsis, Journal of Pineal Research
Galley, Lowes, Allen, Cameron, Aucott et al., Melatonin as a Potential Therapy for Sepsis: A Phase I Dose Escalation Study and an Ex Vivo Whole Blood Model Under Conditions of Sepsis, Journal of Pineal Research
Ganjifard, Ghafari, Sahebnasagh, Evaluation of the Effect of Melatonin on Patients With COVID-19 Admitted to ICU: A Double-Blind Randomized Clinical Trial, Vacunas
Grimshaw, Campbell, Eccles, Steen, Experimental and Quasi-Experimental Designs for Evaluating Guideline Implementation Strategies, Family Practice
Hasan, Atrakji, Mehuaiden, The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients, International Journal of Infectious Diseases
He, Wu, Zhang, Bacteriostatic Potential of Melatonin: Therapeutic Standing and Mechanistic Insights, Frontiers in immunology
Immovilli, Morelli, Rota, Guidetti, COVID-19 Mortality and Health-Care Resources: Organization, Medicina Intensiva
Ji, Ma, Peppelenbosch, Pan, Potential Association Between COVID-19 Mortality and Health-Care Resource Availability, Lancet Global Health
Jiang, Wang, Li, Liver Injury in Critically Ill and Non-Critically Ill COVID-19 Patients: A Multicenter, Retrospective, Observational Study, Frontiers in Medicine
Jou, Peng, Reiter, Jou, Wu et al., Visualization of the Antioxidative Effects of Melatonin at the Mitochondrial Level During Oxidative Stress-Induced Apoptosis of Rat Brain Astrocytes, Journal of Pineal Research
Kireev, Bitoun, Cuesta, Melatonin Treatment Protects Liver of Zucker Rats After Ischemia/Reperfusion by Diminishing Oxidative Stress and Apoptosis, European Journal of Pharmacology
Kireev, Cuesta, Ibarrola, Age-Related Differences in Hepatic Ischemia/Reperfusion: Gene Activation, Liver Injury, and Protective Effect of Melatonin, Journal of Surgical Research
Krisztina Tóth, Landoni, Oreggia, Melatonin as Adjuvant Treatment in COVID-19 Patients. A Meta-Analysis of Randomized and Propensity Matched Studies, Signa Vitae
Lan, Lee, Chao, Chang, Lu et al., Efficacy of Melatonin in the Treatment of Patients With COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Journal of Medical Virology
Lowes, Webster, Murphy, Galley, Antioxidants That Protect Mitochondria Reduce Interleukin-6 and Oxidative Stress, Improve Mitochondrial Function, and Reduce Biochemical Markers of Organ Dysfunction in a Rat Model of Acute Sepsis, British Journal of Anaesthesia
Mistraletti, Paroni, Umbrello, Different Routes and Formulations of Melatonin in Critically Ill Patients. A Pharmacokinetic Randomized Study, Clinical Endocrinology
Mistraletti, Paroni, Umbrello, Melatonin Pharmacological Blood Levels Increase Total Antioxidant Capacity in Critically Ill Patients, International Journal of Molecular Sciences
Mistraletti, Sabbatini, Taverna, Pharmacokinetics of Orally Administered Melatonin in Critically Ill Patients, Journal of Pineal Research
Mistraletti, Umbrello, Sabbatini, Melatonin Reduces the Need for Sedation in ICU Patients: A Randomized Controlled Trial, Minerva Anestesiologica
Mohamed Taha, Adel Abdelkader, Saed, Hossam-Eldin Moawad, Safety and Efficacy of Melatonin as an Adjuvant Therapy in COVID-19 Patients: Systematic Review and Meta-Analysis, Advances in Medical Sciences
Moreno, Rhodes, Piquilloud, The Sequential Organ Failure Assessment (SOFA) Score: Has the Time Come for An Update?, Critical Care
Pearl, Glymor, Jewell, Causal Inference in Statistics: A Primer
Perez-Torres, Aisa-Alvarez, Casarez-Alvarado, Impact of Treatment With Antioxidants as an Adjuvant to Standard Therapy in Patients With Septic Shock: Analysis of the Correlation Between Cytokine Storm and Oxidative Stress and Therapeutic Effects, International Journal of Molecular Sciences
Pham, Le, Pham, Comparative Efficacy of Antioxidant Therapies for Sepsis and Septic Shock in the Intensive Care Unit: A Frequentist Network Meta-Analysis, Heliyon
Phua, Weng, Ling, Intensive Care Management of Coronavirus Disease 2019 (COVID-19): Challenges and Recommendations, Lancet Respiratory Medicine
Pérez-Torres, Merino-García, Canas-Pérez, Real-World Inter-Observer Variability of the Sequential Organ Failure Assessment (SOFA) Score in Intensive Care Medicine: The Time Has Come for an Update, Critical Care
Qin, Feng, Zhou, Bai, Yin, Melatonin Suppresses LPS-Induced Oxidative Stress in Dendritic Cells for Inflammatory Regulation via the Nrf2/HO-1 Axis, Antioxidants
Rodriguez, Mayo, Sainz, Regulation of Antioxidant Enzymes: A Significant Role for Melatonin, Journal of Pineal Research
Shneider, Kudriavtsev, Vakhrusheva, Can Melatonin Reduce the Severity of COVID-19 Pandemic?, International Reviews of Immunology
Steinberger, Finkelstein, Pagano, Barotrauma in COVID 19: Incidence, Pathophysiology, and Effect on Prognosis, Clinical Imaging
Sterne, Murthy, Diaz, Slutsky, Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-Analysis, Journal of the American Medical Association
Tekbas, Ogur, Korkmaz, Kilic, Reiter, Melatonin as an Antibiotic: New Insights Into the Actions of This Ubiquitous Molecule, Journal of Pineal Research
Thorpe, Zwarenstein, Oxman, A Pragmatic-Explanatory Continuum Indicator Summary (PRECIS): A Tool to Help Trial Designers, Journal of Clinical Epidemiology
Tresguerres, Kireev, Forman, Cuesta, Tresguerres et al., Effect of Chronic Melatonin Administration on Several Physiological Parameters From Old Wistar Rats and SAMP8 Mice, Current Aging Science
Vacheron, Lepape, Savey, Attributable Mortality of Ventilator-Associated Pneumonia Among Patients With COVID-19, American Journal of Respiratory and Critical Care Medicine
Van Goethem, Chung, Meurisse, Clinical Severity of SARS-CoV-2 Omicron Variant Compared With Delta Among Hospitalized COVID-19 Patients in Belgium During Autumn and Winter Season 2021-2022, Viruses
Volt, García, Doerrier, Same Molecule but Different Expression: Aging and Sepsis Trigger NLRP3 Inflammasome Activation, a Target of Melatonin, Journal of Pineal Research
Waldhauser, Waldhauser, Lieberman, Deng, Lynch et al., Bioavailability of Oral Melatonin in Humans, Neuroendocrinology
Wolinetz, Tabak, Transforming Clinical Research to Meet Health Challenges, Journal of the American Medical Association
Wongtangman, Santer, Wachtendorf, Association of Sedation, Coma, and In-Hospital Mortality in Mechanically Ventilated Patients With Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study, Critical Care Medicine
Wu, Tsou, Chen, Chen, Tsao et al., Therapeutic Effects of Melatonin on Peritonitis-Induced Septic Shock With Multiple Organ Dysfunction Syndrome in Rats, Journal of Pineal Research
Zhang, Wang, Ni, COVID-19: Melatonin as a Potential Adjuvant Treatment, Life Sciences
DOI record:
{
"DOI": "10.1002/jmv.70807",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.70807",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:p>\n Melatonin has demonstrated antioxidant, anti‐inflammatory, and potential antiviral properties. Its therapeutic role in critically ill COVID‐19 patients admitted to intensive care was underexplored at the start of the pandemic. We conducted a quasi‐experimental, pragmatic study over 4 consecutive uninterrupted time periods alternating control groups receiving standard of care (SoC) with treatment groups receiving SoC plus high‐dose oral bedtime melatonin (50–200 mg) (OBM). The primary endpoint was 90‐day mortality; secondary outcomes included sequential organ failure assessment (SOFA) scores at 4, 7, 14, and 30 days and pre‐defined severe adverse events (SAEs). A total of 335 of 339 consecutive patients with a predicted stay > 48 h were enrolled; 202 received OBM with SoC and 133 received SoC alone. OBM was dispensed during the second (\n <jats:italic>n</jats:italic>\n = 162) and fourth (\n <jats:italic>n</jats:italic>\n = 40) study periods after the first (\n <jats:italic>n</jats:italic>\n = 40) and third (\n <jats:italic>n</jats:italic>\n = 93) control group periods, respectively. Melatonin therapy was associated with significantly lower 90‐day mortality (20.8% vs. 36.1%, OR 0.46, 95% CI 0.28–0.76). Subjects receiving melatonin had lower SOFA scores on Day 4 and subsequent study visits. SAEs occurred in 84 (41.6%) subjects on OBM and in 80 (60.2%) receiving SoC (risk ratio 0.68, 95% CI 0.54–0.87;\n <jats:italic>p</jats:italic>\n = 0.001). High‐dose oral melatonin was safe and associated with improved clinical outcomes. Further evaluation of melatonin and its potential antiviral effects in future epidemics is warranted.\n </jats:p>",
"alternative-id": [
"10.1002/jmv.70807"
],
"article-number": "e70807",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2025-07-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-12-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2026-01-14"
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-8775-255X",
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
},
{
"name": "Department of Medicine, Medical School Universidad Complutense Madrid Madrid Spain"
}
],
"authenticated-orcid": false,
"family": "Sánchez‐García",
"given": "Miguel",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine, Medical School Universidad Complutense Madrid Madrid Spain"
},
{
"name": "Department of Physiology, Medical School Universidad Complutense Madrid Madrid Spain"
}
],
"family": "Tresguerres",
"given": "Jesús A.F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Álvarez‐González",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Hera",
"given": "Belén de la",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Service Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Puebla",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Service Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Ybañez",
"given": "Lidia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Service Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Martínez‐Sesmero",
"given": "José‐Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Blesa",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Martín‐Benítez",
"given": "Juan‐Carlos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Martínez‐Sagasti",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "González‐Arenas",
"given": "Paloma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Domingo",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Pardo",
"given": "Cándido",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Maichle",
"given": "Silmary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Postigo",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "De‐Miguel",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Bringas",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "González‐Casanova",
"given": "Raquel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Yordanov",
"given": "Viktor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Service Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Delgado‐Iribarren",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Service Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Culebras",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Big Data, PMC‐FPS Regional Ministry of Health and Consumer Affairs Sevilla Andalucia Spain"
}
],
"family": "la‐Hoz",
"given": "Miguel A. Armengol‐de",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Hospital Clínico San Carlos Madrid Spain"
}
],
"family": "Núñez‐Reiz",
"given": "Antonio",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T14:34:03Z",
"timestamp": 1768401243000
},
"deposited": {
"date-parts": [
[
2026,
1,
15
]
],
"date-time": "2026-01-15T12:41:29Z",
"timestamp": 1768480889000
},
"indexed": {
"date-parts": [
[
2026,
1,
15
]
],
"date-time": "2026-01-15T22:47:00Z",
"timestamp": 1768517220144,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2026,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2026,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 13,
"start": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T00:00:00Z",
"timestamp": 1768348800000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
1
]
],
"date-time": "2026-01-01T00:00:00Z",
"timestamp": 1767225600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.70807",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jmv.70807",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.70807",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2026,
1
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
14
]
]
},
"published-print": {
"date-parts": [
[
2026,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_11_2_1",
"unstructured": "“Centro‐de‐Coordinación‐de‐Alertas‐y‐Emergencias‐Sanitarias. Actualización n° 58. Enfermedad por el coronavirus (COVID‐19) ” [Online Document]. Madrid: Ministerio de Sanidad. Madrid; 2020 [updated 28/03/2020; cited 2024 12/10/2024]. 1‐10] https://www.mclibre.org/descargar/informatica/covid-19/covid-19-058-20200328.pdf."
},
{
"DOI": "10.1056/NEJMoa2004500",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.1007/s00134-020-06092-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1111/j.1600-079X.2004.00140.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1111/j.1600-079X.2005.00265.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.2174/1874609811205030012",
"article-title": "Effect of Chronic Melatonin Administration on Several Physiological Parameters From Old Wistar Rats and SAMP8 Mice",
"author": "A.F. Tresguerres J.",
"doi-asserted-by": "crossref",
"first-page": "242",
"issue": "3",
"journal-title": "Current Aging Science",
"key": "e_1_2_11_7_1",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.1111/jpi.12303",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.3390/ijms18040759",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1016/j.ejphar.2012.11.038",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1016/j.jss.2012.04.060",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1002/jcph.592",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.21037/jtd.2019.12.85",
"article-title": "Melatonin for the Treatment of Sepsis: The Scientific Rationale",
"author": "Colunga Biancatelli R. M. L.",
"doi-asserted-by": "crossref",
"first-page": "S54",
"issue": "1",
"journal-title": "Journal of Thoracic Disease",
"key": "e_1_2_11_13_1",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117583",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1002/rmv.2109",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1080/08830185.2020.1756284",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1097/CCM.0000000000004363",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1056/NEJMra1510059",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1016/j.jclinepi.2008.12.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1016/S2213-2600(20)30161-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1016/j.medin.2020.04.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"key": "e_1_2_11_22_1",
"unstructured": "“Centro de Coordinación de Alertas y Emergencias Sanitarias Dirección General de Salud Pública CeI Manejo Clínico Del COVID‐19: Unidades De Cuidados Intensivos Madrid: Ministerio de Sanidad ” 2020 https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Protocolo_manejo_clinico_uci_COVID-19.pdf."
},
{
"DOI": "10.1159/000123997",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.1111/jpi.12134",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1186/s40360-016-0052-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1111/cen.13993",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"DOI": "10.1007/s40262-016-0386-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"author": "Pearl J.",
"first-page": "160",
"key": "e_1_2_11_28_1",
"volume-title": "Causal Inference in Statistics: A Primer",
"year": "2016"
},
{
"DOI": "10.1016/S2214-109X(20)30068-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.1016/j.medin.2020.05.014",
"article-title": "COVID‐19 Mortality and Health‐Care Resources: Organization",
"author": "Immovilli P.",
"doi-asserted-by": "crossref",
"first-page": "383",
"issue": "6",
"journal-title": "Medicina Intensiva",
"key": "e_1_2_11_30_1",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"DOI": "10.1111/jpi.12830",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"DOI": "10.3390/v14061297",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"article-title": "Acute Respiratory Distress Syndrome: The Berlin Definition",
"author": "ARDS Definition Task F.",
"first-page": "2526",
"issue": "23",
"journal-title": "Journal of the American Medical Association",
"key": "e_1_2_11_34_1",
"volume": "307",
"year": "2012"
},
{
"DOI": "10.1002/jmv.27595",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.1016/j.advms.2023.09.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"article-title": "Melatonin as Adjuvant Treatment in COVID‐19 Patients. A Meta‐Analysis of Randomized and Propensity Matched Studies",
"author": "Krisztina Tóth E. P.",
"first-page": "8",
"issue": "1",
"journal-title": "Signa Vitae",
"key": "e_1_2_11_37_1",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1111/j.1600-079X.2008.00567.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"article-title": "Melatonin Reduces the Need for Sedation in ICU Patients: A Randomized Controlled Trial",
"author": "Mistraletti G.",
"first-page": "1298",
"issue": "12",
"journal-title": "Minerva Anestesiologica",
"key": "e_1_2_11_39_1",
"volume": "81",
"year": "2015"
},
{
"DOI": "10.1111/j.1600-079X.2009.00737.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.1016/j.csbj.2021.02.009",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"DOI": "10.3390/ijms242316610",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_42_1"
},
{
"DOI": "10.1016/j.heliyon.2024.e31447",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_43_1"
},
{
"DOI": "10.1186/s13054-022-04290-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
},
{
"DOI": "10.1186/s13054-023-04449-y",
"article-title": "Real‐World Inter‐Observer Variability of the Sequential Organ Failure Assessment (SOFA) Score in Intensive Care Medicine: The Time Has Come for an Update",
"author": "Pérez‐Torres D.",
"doi-asserted-by": "crossref",
"first-page": "160",
"issue": "1",
"journal-title": "Critical Care",
"key": "e_1_2_11_45_1",
"volume": "27",
"year": "2023"
},
{
"DOI": "10.1007/s10787-022-01096-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1164/rccm.202202-0357OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
},
{
"DOI": "10.1111/j.1600-079X.2007.00516.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_48_1"
},
{
"DOI": "10.3389/fimmu.2021.683879",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_49_1"
},
{
"DOI": "10.1016/j.clinimag.2022.06.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_50_1"
},
{
"DOI": "10.1097/CCM.0000000000005053",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_51_1"
},
{
"DOI": "10.3389/fmed.2020.00347",
"article-title": "Liver Injury in Critically Ill and Non‐Critically Ill COVID‐19 Patients: A Multicenter, Retrospective, Observational Study",
"author": "Jiang S.",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "Frontiers in Medicine",
"key": "e_1_2_11_52_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.vacun.2024.09.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_53_1"
},
{
"DOI": "10.1093/fampra/17.suppl_1.S11",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_54_1"
},
{
"DOI": "10.1016/S2213-2600(22)00044-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_55_1"
},
{
"DOI": "10.1002/pam.22051",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_56_1"
},
{
"DOI": "10.1136/pgmj.2004.026807",
"article-title": "Limitations of ‘Pragmatic’ Trials",
"author": "Ernst E.",
"doi-asserted-by": "crossref",
"first-page": "203",
"issue": "954",
"journal-title": "Postgraduate Medical Journal",
"key": "e_1_2_11_57_1",
"volume": "81",
"year": "2005"
},
{
"DOI": "10.1001/jama.2023.3964",
"article-title": "Transforming Clinical Research to Meet Health Challenges",
"author": "Wolinetz C. D.",
"doi-asserted-by": "crossref",
"first-page": "1740",
"journal-title": "Journal of the American Medical Association",
"key": "e_1_2_11_58_1",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1046/j.1600-079X.2003.00092.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_59_1"
},
{
"DOI": "10.1093/bja/aes577",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_60_1"
},
{
"DOI": "10.1016/j.ijid.2021.10.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_61_1"
},
{
"DOI": "10.3390/antiox11102012",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_62_1"
}
],
"reference-count": 61,
"references-count": 61,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.70807"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Oral Melatonin in Critically Ill Patients With COVID‐19: A Quasi‐Experimental Pragmatic Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "98"
}