Outcome of Different Therapeutic Interventions in Mild COVID-19 Patients in a Single OPD Clinic of West Bengal: A Retrospective study
et al., medRxiv, doi:10.1101/2021.03.08.21252883, Mar 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
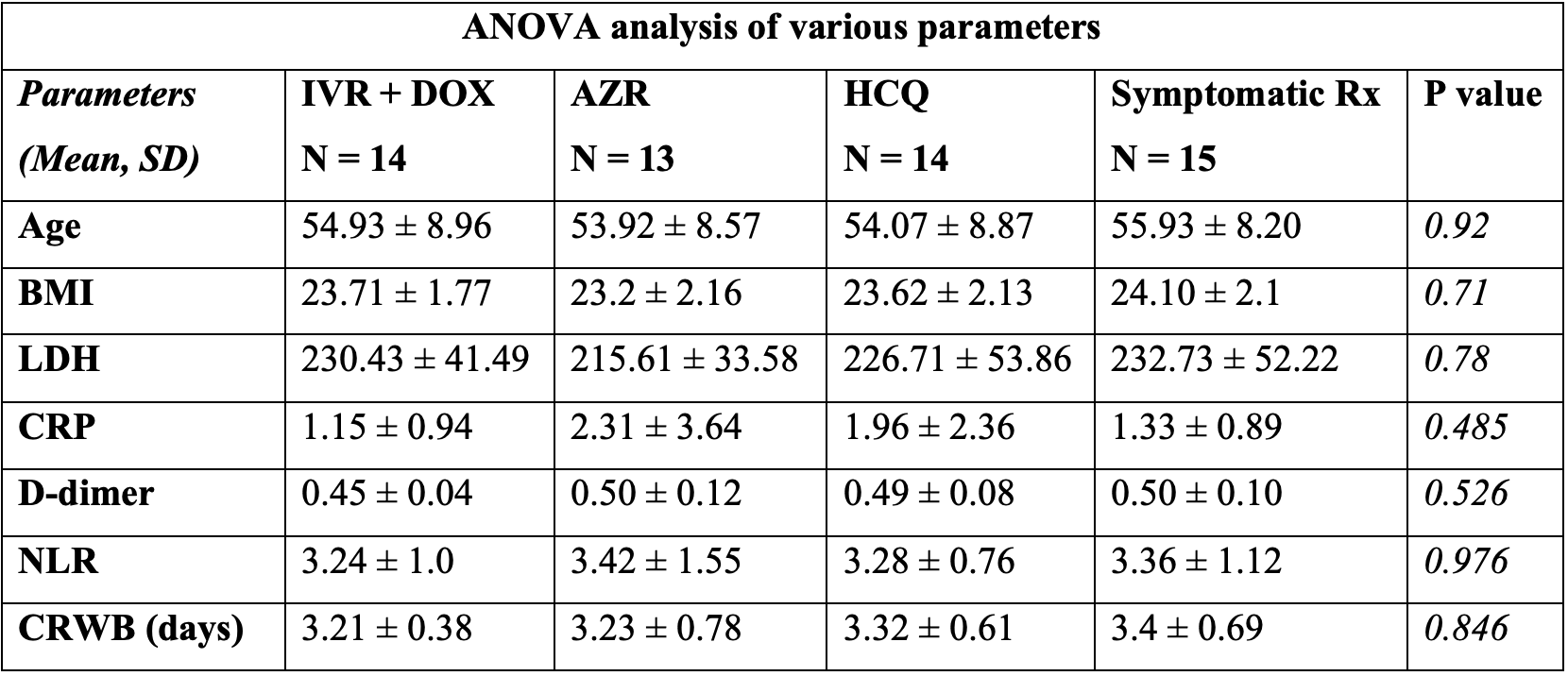
Retrospective database analysis of 56 mild COVID-19 patients, all treated with vitamin C, vitamin D, and zinc, comparing ivermectin + doxycycline (n=14), AZ (n=13), HCQ (n=14), and SOC (n=15), finding that all groups recover quickly, and there was no significant difference between the groups.
This study has severe selection bias due to post-treatment exclusion based on outcomes. Authors excluded 10 patients who were hospitalized for "new-onset breathlessness" during follow-up - hospitalization being a key clinical outcome of COVID-19 treatment. By removing treatment failures from the analysis, the study only compares patients who responded well enough to remain at home. Critically, the distribution of these 10 hospitalizations across treatment arms was not reported.
Moreover, this study has multiple unresolved data integrity issues that persisted across two preprint versions and the peer-reviewed publication. Critical concerns include: (1) an unexplained approximately two-fold discrepancy in reported standard deviations for age and BMI between Table 1 and Table 2, with no valid statistical explanation for this systematic difference; (2) an error in the azithromycin group where the reported gender ratio does not match the stated sample size (n=13); (3) multiple GRIM test failures indicating that reported means for the primary outcome (CRWB) and age are mathematically impossible given the sample sizes and integer-valued measurements; and (4) an implausibly low coefficient of variation for D-dimer in the ivermectin+doxycycline group compared to other groups, suggesting data anomalies. These errors remained uncorrected in the journal publication, raising concerns about data verifiability. The study also describes prospective consent collection despite claiming to be a retrospective database analysis.
The study also lacks trial registration and reports unadjusted results while multiple baseline variables have 25+% difference between groups.
This is the 43rd of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
53 studies are RCTs, which show efficacy with p=0.000000087.
This study is excluded in the after exclusion results of meta-analysis:
no serious outcomes reported and fast recovery in treatment and control groups, there is little room for a treatment to improve results.
Study covers HCQ and ivermectin.
|
relative time to clinical response of wellbeing, 5.6% lower, relative time 0.94, p = 0.87, treatment 14, control 15, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Roy et al., 12 Mar 2021, retrospective, database analysis, India, preprint, 5 authors, dosage not specified, this trial uses multiple treatments in the treatment arm (combined with doxycycline) - results of individual treatments may vary.
Outcome of Different Therapeutic Interventions in Mild COVID-19 Patients in a Single OPD Clinic of West Bengal: A Retrospective study
doi:10.1101/2021.03.08.21252883
Introduction: With over 87,273,380 cases being reported and 1,899,440 deaths worldwide as of 9th January 2021, Coronavirus disease 2019 (COVID-19) has become the worst-hit pandemic till date. Every day clinicians are bombarded with many new treatment options that claim to be better than the others.
References
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Ciccullo, Borghetti, Dal Verme, Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line, Int J Antimicrob Agents
Healthworld, COVID-19: Health Ministry issues revised home isolation guidelines
Janowitz, Gablenz, Pattinson, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut
Liu, Cao, Xu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Ncid, None
Oldenburg, Doan, Azithromycin for severe COVID-19, Lancet
Roy, Journey So Far With COVID 19 -A Comprehensive Review, European Journal Of Clinical And Experimental Medicine
Roy, Mainakmukhopadhyay, Ventricular arrhythmia risk based on ethnicity in COVID-19 patients on hydroxychloroquine and azithromycin combination: Viewpoint, SN Compr Clin Med
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Summary, Therapeutic management of patients with COVID-19
Velavan, Meyer, Mild versus severe COVID-19: Laboratory markers, Int J Infect Dis
Who, None
Wu, Liu, Yang, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharm Sin B
Zamanian, Pollack, Jr, Gentile, Outpatient inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 infection, Am J Respir Crit Care Med
DOI record:
{
"DOI": "10.1101/2021.03.08.21252883",
"URL": "http://dx.doi.org/10.1101/2021.03.08.21252883",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Introduction</jats:title><jats:p>With over 87,273,380 cases being reported and 1,899,440 deaths worldwide as of 9th January 2021, Coronavirus disease 2019 (COVID-19) has become the worst-hit pandemic till date. Every day clinicians are bombarded with many new treatment options that claim to be better than the others.</jats:p></jats:sec><jats:sec><jats:title>Materials and methods</jats:title><jats:p>After screening the electronic database of COVID-19 patients retrospectively, 56 patients with mild COVID-19 infection matched the inclusion criteria and were divided into the four following groups - group having used Hydroxychloroquine (HCQ), group using doxycycline (DOX) + Ivermectin (IVR) combination, group receiving only azithromycin (AZ) and, group receiving only symptomatic treatment. The study’s primary objective was to see Clinical response of well-being (CRWB) reporting time after initiating treatment onset between the four different treatment arms.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>CRWB did not differ between the four groups receiving four different managements (p-value 0.846). There was significant correlation between blood levels of LDH (p-value 0.001), CRP (p-value 0.03) and D-dimer (p-value 0.04) with CRWB in IVR+DOX group and, between LDH (p-value 0.001), CRP (p-value 0.01) and age (p-value 0.035) with CRWB in the symptomatic management group.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Mild COVID-19 infection in patients having low-risk to progress can be managed symptomatically without any specific drug intervention.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
3,
22
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6185-9375",
"affiliation": [],
"authenticated-orcid": false,
"family": "Roy",
"given": "Sayak",
"sequence": "first"
},
{
"affiliation": [],
"family": "Samajdar",
"given": "Shambo Samrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tripathi",
"given": "Santanu K",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9524-1525",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mukherjee",
"given": "Shatavisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhattacharjee",
"given": "Kingshuk",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
12
]
],
"date-time": "2021-03-12T14:40:23Z",
"timestamp": 1615560023000
},
"deposited": {
"date-parts": [
[
2022,
12,
21
]
],
"date-time": "2022-12-21T09:03:55Z",
"timestamp": 1671613435000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
12,
22
]
],
"date-time": "2022-12-22T05:31:07Z",
"timestamp": 1671687067555
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2021,
3,
12
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.03.08.21252883",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
3,
12
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
3,
12
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021032410001188000_2021.03.08.21252883v2.1",
"unstructured": "Who.int. Accessed January 16, 2021. https://covid19.who.int/."
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.2",
"unstructured": "Executive Summary. Therapeutic management of patients with COVID-19. Nih.gov. Accessed January 16, 2021. https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_100.pdf"
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.3",
"unstructured": "Gov.in. Accessed January 16, 2021. https://www.wbhealth.gov.in/uploaded_files/corona/WB_Covid_protocol_book_25.09_.20_(1)_.pdf"
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.4",
"unstructured": "HealthWorld ET. COVID-19: Health Ministry issues revised home isolation guidelines. PTI. Published July 3, 2020. Accessed January 16, 2021. https://health.economictimes.indiatimes.com/news/policy/covid-19-health-ministry-issues-revised-home-isolation-guidelines/76767874"
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.5",
"unstructured": "Clinical management of COVID-19. Who.int. Accessed January 16, 2021. https://www.who.int/publications/i/item/clinical-management-of-covid-19"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"doi-asserted-by": "publisher",
"key": "2021032410001188000_2021.03.08.21252883v2.6"
},
{
"article-title": "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"first-page": "214",
"journal-title": "Int J Infect Dis",
"key": "2021032410001188000_2021.03.08.21252883v2.7",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202004-0937LE",
"article-title": "Outpatient inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 infection",
"doi-asserted-by": "crossref",
"first-page": "130",
"issue": "1",
"journal-title": "Am J Respir Crit Care Med",
"key": "2021032410001188000_2021.03.08.21252883v2.8",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"article-title": "Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods",
"doi-asserted-by": "crossref",
"first-page": "766",
"issue": "5",
"journal-title": "Acta Pharm Sin B",
"key": "2021032410001188000_2021.03.08.21252883v2.9",
"volume": "10",
"year": "2020"
},
{
"article-title": "MainakMukhopadhyay. Ventricular arrhythmia risk based on ethnicity in COVID-19 patients on hydroxychloroquine and azithromycin combination: Viewpoint",
"first-page": "1",
"issue": "8",
"journal-title": "SN Compr Clin Med",
"key": "2021032410001188000_2021.03.08.21252883v2.10",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31863-8",
"article-title": "Azithromycin for severe COVID-19",
"doi-asserted-by": "crossref",
"first-page": "936",
"issue": "10256",
"journal-title": "Lancet",
"key": "2021032410001188000_2021.03.08.21252883v2.11",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"doi-asserted-by": "publisher",
"key": "2021032410001188000_2021.03.08.21252883v2.12"
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.13",
"unstructured": "Ncid.sg. Accessed January 16, 2021. https://www.ncid.sg/Health-Professionals/Diseases-and-Conditions/Documents/Treatment%20Guidelines%20for%20COVID-19%20%282%20Apr%202020%29%20-final.pdf"
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.14",
"unstructured": "Therapeutic Management. Nih.gov. Accessed January 16, 2021. https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/"
},
{
"DOI": "10.15584/ejcem.2020.4.7",
"doi-asserted-by": "crossref",
"key": "2021032410001188000_2021.03.08.21252883v2.15",
"unstructured": "Roy, S. , 2020. Journey So Far With COVID 19 – A Comprehensive Review | European Journal Of Clinical And Experimental Medicine. [online] Ejcem.ur.edu.pl. Available at: <http://www.ejcem.ur.edu.pl/summary/journey-so-far-covid-19-comprehensive-review> [accessed 17 January 2021]. http://www.ejcem.ur.edu.pl/summary/journey-so-far-covid-19-comprehensive-review"
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.16",
"unstructured": "Prophylactic Ivermectin in COVID-19 Contacts. Clinicaltrials.gov. Accessed January 16, 2021. https://clinicaltrials.gov/ct2/show/results/NCT04422561."
},
{
"key": "2021032410001188000_2021.03.08.21252883v2.17",
"unstructured": "USEFULNESS of Topic Ivermectin and Carrageenan to Prevent Contagion of Covid 19 - Study Results - ClinicalTrials.Gov. Clinicaltrials.gov. Accessed January 16, 2021. https://clinicaltrials.gov/ct2/show/results/NCT04425850."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106017",
"article-title": "Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line",
"doi-asserted-by": "crossref",
"first-page": "106017",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "2021032410001188000_2021.03.08.21252883v2.18",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.04.061",
"doi-asserted-by": "publisher",
"key": "2021032410001188000_2021.03.08.21252883v2.19"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"doi-asserted-by": "publisher",
"key": "2021032410001188000_2021.03.08.21252883v2.20"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.03.08.21252883"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Outcome of Different Therapeutic Interventions in Mild COVID-19 Patients in a Single OPD Clinic of West Bengal: A Retrospective study",
"type": "posted-content"
}
