Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study
et al., QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcaa206, Jun 2020
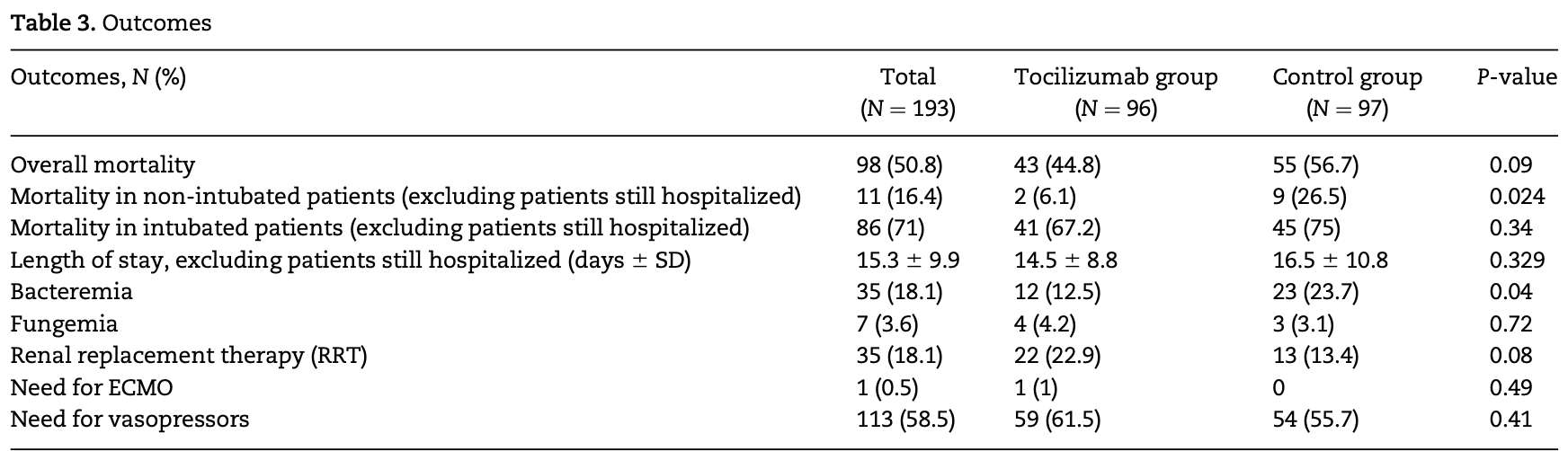
Retrospective case-control study of 193 hospitalized COVID-19 patients showing a non-statistically significant trend toward lower mortality with tocilizumab treatment. When excluding intubated patients, tocilizumab was associated with significantly lower mortality.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 21.0% lower, RR 0.79, p = 0.11, treatment 43 of 96 (44.8%), control 55 of 97 (56.7%), NNT 8.4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rojas-Marte et al., 19 Jun 2020, retrospective, USA, peer-reviewed, mean age 60.0, 18 authors, study period 8 March, 2020 - 25 April, 2020.
Contact: grmarte@maimonidesmed.org.
Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study
QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcaa206
Background: COVID-19 is an ongoing threat to society. Patients who develop the most severe forms of the disease have high mortality. The interleukin-6 inhibitor tocilizumab has the potential to improve outcomes in these patients by preventing the development of cytokine release storm. Aims: To evaluate the outcomes of patients with severe COVID-19 disease treated with the interleukin-6 inhibitor tocilizumab. Methods: We conducted a retrospective, case-control, single-center study in patients with severe to critical COVID-19 disease treated with tocilizumab. Disease severity was defined based on the amount of oxygen supplementation required. The primary endpoint was the overall mortality. Secondary endpoints were mortality in non-intubated patients and mortality in intubated patients. Results: A total of 193 patients were included in the study. Ninety-six patients received tocilizumab, while 97 served as the control group. The mean age was 60 years. Patients over 65 years represented 43% of the population. More patients in the tocilizumab group reported fever, cough and shortness of breath (83%, 80% and 96% vs. 73%, 69% and 71%, respectively). There was a non-statistically significant lower mortality in the treatment group (52% vs. 62.1%, P ¼ 0.09). When excluding intubated patients, there was statistically significant lower mortality in patients treated with tocilizumab (6% vs. 27%, P ¼ 0.024). Bacteremia was more common in the control group (24% vs. 13%, P ¼ 0.43), while fungemia was similar for both (3% vs. 4%, P ¼ 0.72). Conclusion: Our study showed a non-statistically significant lower mortality in patients with severe to critical COVID-19 disease who received tocilizumab. When intubated patients were excluded, the use of tocilizumab was associated with lower mortality.
Conflict of interest: The authors declare that there is no conflict of interest regarding the publication of this article. .
References
Benedetti, Brunner, Ruperto, Kenwright, Wright et al., Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis, N Engl J Med
Capra, Rossi, Mattioli, Romanelli, Scarpazza et al., Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia, Eur J Intern Med
Center, Johns Hopkins Coronavirus Resource Center
Channappanavar, Perlman, Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Sem Immunopathol
Colaneri, Bogliolo, Valsecchi, Sacchi, Zuccaro et al., COVID IRCCS San Matteo Pavia Task Force. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE), Microorganisms
Fu, Xu, Wei, Why tocilizumab could be an effective treatment for severe COVID-19?, J Transl Med
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy, JAMA
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jani, Barton, Hyrich, Prediction of infection risk in rheumatoid arthritis patients treated with biologics: are we any closer to risk stratification?, Curr Opin Rheumatol
Klopfenstein, Zayet, Lohse, Balblanc, Badie et al., Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients, Med Mal Infect
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)induced cytokine release syndrome (CRS)?, J Autoimmun
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: a single center experience, J Med Virol
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Toniati, Piva, Cattalini, Garrafa, Regola et al., Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy, Autoimmun Rev
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention, JAMA
Xu, Han, Li, Sun, Wang et al., Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci
Yang, Yu, Xu, Shu, Xia et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med
Zheng, Peng, Xu, Zhao, Liu et al., Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.04.021
DOI record:
{
"DOI": "10.1093/qjmed/hcaa206",
"ISSN": [
"1460-2725",
"1460-2393"
],
"URL": "http://dx.doi.org/10.1093/qjmed/hcaa206",
"abstract": "<jats:title>Summary</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>COVID-19 is an ongoing threat to society. Patients who develop the most severe forms of the disease have high mortality. The interleukin-6 inhibitor tocilizumab has the potential to improve outcomes in these patients by preventing the development of cytokine release storm.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Aims</jats:title>\n <jats:p>To evaluate the outcomes of patients with severe COVID-19 disease treated with the interleukin-6 inhibitor tocilizumab.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a retrospective, case–control, single-center study in patients with severe to critical COVID-19 disease treated with tocilizumab. Disease severity was defined based on the amount of oxygen supplementation required. The primary endpoint was the overall mortality. Secondary endpoints were mortality in non-intubated patients and mortality in intubated patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 193 patients were included in the study. Ninety-six patients received tocilizumab, while 97 served as the control group. The mean age was 60 years. Patients over 65 years represented 43% of the population. More patients in the tocilizumab group reported fever, cough and shortness of breath (83%, 80% and 96% vs. 73%, 69% and 71%, respectively). There was a non-statistically significant lower mortality in the treatment group (52% vs. 62.1%, P = 0.09). When excluding intubated patients, there was statistically significant lower mortality in patients treated with tocilizumab (6% vs. 27%, P = 0.024). Bacteremia was more common in the control group (24% vs. 13%, P = 0.43), while fungemia was similar for both (3% vs. 4%, P = 0.72).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Our study showed a non-statistically significant lower mortality in patients with severe to critical COVID-19 disease who received tocilizumab. When intubated patients were excluded, the use of tocilizumab was associated with lower mortality.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "https://orcid.org/0000-0001-7536-3090",
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
},
{
"name": "Department of Cardiology, Staten Island University Hospital-Northwell Health, 475 Seaview Avenue, Staten Island, NY 10305, USA"
}
],
"authenticated-orcid": false,
"family": "Rojas-Marte",
"given": "G",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Khalid",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonology, Interfaith Medical Center, 1545 Atlantic Avenue, Brooklyn, NY 11213, USA"
}
],
"family": "Mukhtar",
"given": "O",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3205-5658",
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"authenticated-orcid": false,
"family": "Hashmi",
"given": "A T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Waheed",
"given": "M A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Ehrlich",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Aslam",
"given": "A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Siddiqui",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Agarwal",
"given": "C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Malyshev",
"given": "Y",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Henriquez-Felipe",
"given": "C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Sharma",
"given": "D",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6039-5735",
"affiliation": [
{
"name": "Department of Internal Medicine, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"authenticated-orcid": false,
"family": "Sharma",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Chukwuka",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Rodriguez",
"given": "D C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Alliu",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Le",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA"
}
],
"family": "Shani",
"given": "J",
"sequence": "additional"
}
],
"container-title": "QJM: An International Journal of Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
6,
12
]
],
"date-time": "2020-06-12T11:31:04Z",
"timestamp": 1591961464000
},
"deposited": {
"date-parts": [
[
2020,
8,
8
]
],
"date-time": "2020-08-08T02:09:52Z",
"timestamp": 1596852592000
},
"indexed": {
"date-parts": [
[
2025,
5,
27
]
],
"date-time": "2025-05-27T17:08:17Z",
"timestamp": 1748365697435,
"version": "3.37.3"
},
"is-referenced-by-count": 103,
"issue": "8",
"issued": {
"date-parts": [
[
2020,
6,
19
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2020,
6,
19
]
]
},
"published-print": {
"date-parts": [
[
2020,
8,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
19
]
],
"date-time": "2020-06-19T00:00:00Z",
"timestamp": 1592524800000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/qjmed/advance-article-pdf/doi/10.1093/qjmed/hcaa206/33445195/hcaa206.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/qjmed/article-pdf/113/8/546/33590042/hcaa206.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/qjmed/article-pdf/113/8/546/33590042/hcaa206.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "546-550",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2020,
6,
19
]
]
},
"published-online": {
"date-parts": [
[
2020,
6,
19
]
]
},
"published-other": {
"date-parts": [
[
2020,
8
]
]
},
"published-print": {
"date-parts": [
[
2020,
8,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"key": "2020080722064420500_hcaa206-B1"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "2020080722064420500_hcaa206-B2",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"article-title": "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "475",
"journal-title": "Lancet Respir Med",
"key": "2020080722064420500_hcaa206-B3",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.04.021",
"article-title": "Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis",
"author": "Zheng",
"doi-asserted-by": "crossref",
"journal-title": "J Infect",
"key": "2020080722064420500_hcaa206-B4",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1574",
"journal-title": "Italy. JAMA",
"key": "2020080722064420500_hcaa206-B5",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "2020080722064420500_hcaa206-B6",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "10970",
"journal-title": "Proc Natl Acad Sci USA",
"key": "2020080722064420500_hcaa206-B7",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1007/s00281-017-0629-x",
"article-title": "Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology",
"author": "Channappanavar",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": ". Sem Immunopathol",
"key": "2020080722064420500_hcaa206-B8",
"volume": "39",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "China. Lancet",
"key": "2020080722064420500_hcaa206-B9",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"article-title": "Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "102452",
"journal-title": "J Autoimmun",
"key": "2020080722064420500_hcaa206-B10",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25801",
"article-title": "Tocilizumab treatment in COVID-19: a single center experience",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "814",
"journal-title": "J Med Virol",
"key": "2020080722064420500_hcaa206-B11",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1186/s12967-020-02339-3",
"article-title": "Why tocilizumab could be an effective treatment for severe COVID-19?",
"author": "Fu",
"doi-asserted-by": "crossref",
"first-page": "164",
"journal-title": "J Transl Med",
"key": "2020080722064420500_hcaa206-B12",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.009",
"article-title": "Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia",
"author": "Capra",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Eur J Intern Med",
"key": "2020080722064420500_hcaa206-B13",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/j.medmal.2020.05.001",
"article-title": "Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients",
"author": "Klopfenstein",
"doi-asserted-by": "crossref",
"journal-title": "Med Mal Infect",
"key": "2020080722064420500_hcaa206-B14",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"article-title": "Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia",
"author": "Toniati",
"doi-asserted-by": "crossref",
"first-page": "102568",
"journal-title": "Italy. Autoimmun Rev",
"key": "2020080722064420500_hcaa206-B15",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.3390/microorganisms8050695",
"article-title": "Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE)",
"author": "Colaneri",
"doi-asserted-by": "crossref",
"first-page": "695",
"journal-title": "Microorganisms",
"key": "2020080722064420500_hcaa206-B16",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1112802",
"article-title": "Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis",
"author": "De Benedetti",
"doi-asserted-by": "crossref",
"first-page": "2385",
"journal-title": "N Engl J Med",
"key": "2020080722064420500_hcaa206-B17",
"volume": "367",
"year": "2012"
},
{
"DOI": "10.1097/BOR.0000000000000598",
"article-title": "Prediction of infection risk in rheumatoid arthritis patients treated with biologics: are we any closer to risk stratification?",
"author": "Jani",
"doi-asserted-by": "crossref",
"first-page": "285",
"journal-title": "Curr Opin Rheumatol",
"key": "2020080722064420500_hcaa206-B18",
"volume": "31",
"year": "2019"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/qjmed/article/113/8/546/5860840"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study",
"type": "journal-article",
"volume": "113"
}