A randomized, double-blind study on the efficacy of oral domperidone versus placebo for reducing SARS-CoV-2 viral load in mild-to-moderate COVID-19 patients in primary health care
et al., Annals of Medicine, doi:10.1080/07853890.2023.2268535, Oct 2023
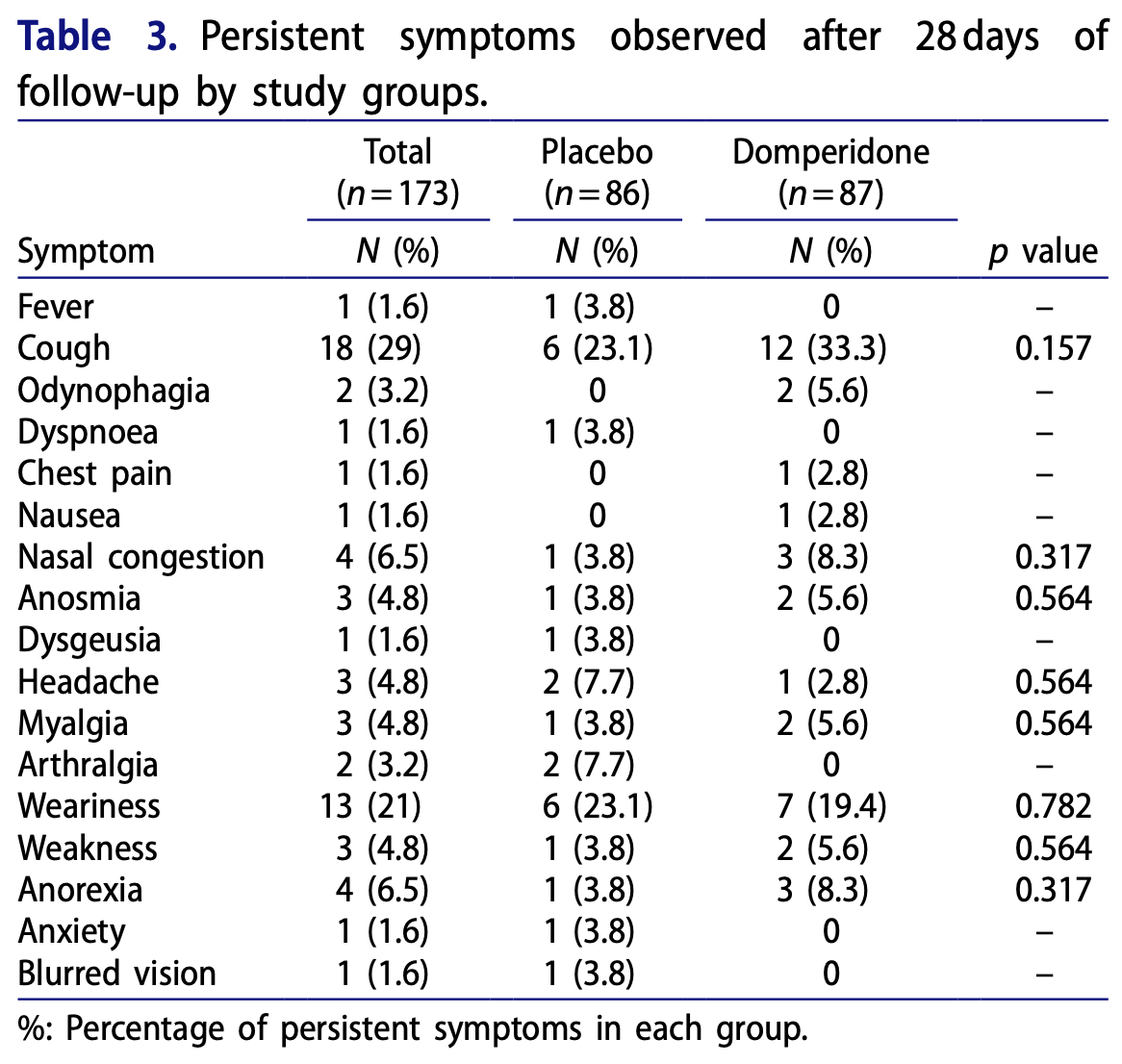
RCT 173 outpatients with mild-to-moderate COVID-19 showing no significant difference with domperidone versus placebo for viral clearance or symptom recovery.
|
risk of no recovery, 36.0% higher, RR 1.36, p = 0.23, treatment 87, control 86, all symptoms.
|
|
risk of no recovery, 66.8% lower, RR 0.33, p = 0.50, treatment 0 of 87 (0.0%), control 1 of 86 (1.2%), NNT 86, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28, fever.
|
|
risk of no recovery, 97.7% higher, RR 1.98, p = 0.21, treatment 12 of 87 (13.8%), control 6 of 86 (7.0%), day 28, cough.
|
|
risk of no recovery, 397.7% higher, RR 4.98, p = 0.50, treatment 2 of 87 (2.3%), control 0 of 86 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28, odynophagia.
|
|
risk of no recovery, 66.8% lower, RR 0.33, p = 0.50, treatment 0 of 87 (0.0%), control 1 of 86 (1.2%), NNT 86, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28, dyspnea.
|
|
risk of no recovery, 198.9% higher, RR 2.99, p = 1.00, treatment 1 of 87 (1.1%), control 0 of 86 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28, chest pain.
|
|
risk of no recovery, 198.9% higher, RR 2.99, p = 1.00, treatment 1 of 87 (1.1%), control 0 of 86 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28, nausea.
|
|
risk of no recovery, 196.6% higher, RR 2.97, p = 0.62, treatment 3 of 87 (3.4%), control 1 of 86 (1.2%), day 28, nasal congestion.
|
|
risk of no recovery, 97.7% higher, RR 1.98, p = 1.00, treatment 2 of 87 (2.3%), control 1 of 86 (1.2%), day 28, anosmia.
|
|
risk of no recovery, 66.8% lower, RR 0.33, p = 0.50, treatment 0 of 87 (0.0%), control 1 of 86 (1.2%), NNT 86, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28, dysgeusia.
|
|
risk of no recovery, 50.6% lower, RR 0.49, p = 0.62, treatment 1 of 87 (1.1%), control 2 of 86 (2.3%), NNT 85, day 28, headache.
|
|
risk of no recovery, 97.7% higher, RR 1.98, p = 1.00, treatment 2 of 87 (2.3%), control 1 of 86 (1.2%), day 28, myalgia.
|
|
risk of no recovery, 80.1% lower, RR 0.20, p = 0.25, treatment 0 of 87 (0.0%), control 2 of 86 (2.3%), NNT 43, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28, arthralgia.
|
|
risk of no recovery, 15.3% higher, RR 1.15, p = 1.00, treatment 7 of 87 (8.0%), control 6 of 86 (7.0%), day 28, weariness.
|
|
risk of no recovery, 97.7% higher, RR 1.98, p = 1.00, treatment 2 of 87 (2.3%), control 1 of 86 (1.2%), day 28, weakness.
|
|
risk of no recovery, 196.6% higher, RR 2.97, p = 0.62, treatment 3 of 87 (3.4%), control 1 of 86 (1.2%), day 28, anorexia.
|
|
risk of no recovery, 66.8% lower, RR 0.33, p = 0.50, treatment 0 of 87 (0.0%), control 1 of 86 (1.2%), NNT 86, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28, anxiety.
|
|
risk of no recovery, 66.8% lower, RR 0.33, p = 0.50, treatment 0 of 87 (0.0%), control 1 of 86 (1.2%), NNT 86, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28, blurred vision.
|
|
risk of no viral clearance, 15.3% higher, RR 1.15, p = 0.62, treatment 28 of 87 (32.2%), control 24 of 86 (27.9%), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rabanal Basalo et al., 17 Oct 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, mean age 47.8, 39 authors, study period 22 March, 2022 - 3 November, 2022.
Contact: carmen.gil@csic.es, ana.martinez@csic.es, bsoler@ecbio.net.
A randomized, double-blind study on the efficacy of oral domperidone versus placebo for reducing SARS-CoV-2 viral load in mild-to-moderate COVID-19 patients in primary health care
Annals of Medicine, doi:10.1080/07853890.2023.2268535
Introduction: the clinical effect of domperidone against cOViD-19 has been investigated in a double-blind phase iii clinical trial (eudract number 2021-001228-17). Domperidone has shown in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2 (saRs-coV-2) and potential immudolatory properties through the stimulation of prolactin secretion. Patients and methods: the efficacy of oral domperidone plus standard of care (sOc; n = 87) versus placebo plus sOc (n = 86) was evaluated in a 28-day randomized double-blind multicentre study in primary health care centres. a total of 173 outpatients with mild-tomoderate cOViD-19 were included. three daily doses of 10 mg (30 mg/day) of domperidone or placebo were administered for 7 days. Reduction of viral load on day 4 was the primary efficay endpoint. it was estimated in saliva samples by reverse transcription-quantitative polymerase chain reaction (Rt-qPcR), as the cycle thresholds detected ORF1ab, N Protein and s Protein genes. Results: a significant reduction in the viral load was observed (p < 0.001) from baseline to days 4, 7 and 14 of the three genes studied with non-significant differences between domperidone and placebo groups. twenty-three patients (13.3%) experienced adverse events, 14 patients in the domperidone group (16.1%) and 9 patients in the placebo group (10.5%). No patients needed to be hospitalized.
Conclusion: Results do not prove the use of domperidone as antiviral in patients with cOViD-19.
Authors contributions
Disclosure statement a. Martínez and c. Gil are employees of the sponsor agencia estatal consejo superior de investigaciones científicas, M.P. (csic). B. soler lópez was contracted to carry out the design, monitoring, statistical analysis and management of the publications derived from the study. the other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Baker Ba, Tj, Plasma prolactin and clinical outcome in preterm infants, arch Dis child, doi:10.1136/adc.65.9.977
Bellis, Pivonello, Prolactin and autoimmunity, Pituitary, doi:10.1007/s11102-005-5082-5
Borba, Zandman-Goddard, Prolactin and autoimmunity, Front immunol, doi:10.3389/fimmu.2018.00073
Drożdżal S, Rosik, an update on drugs with therapeutic potential for saRs-coV-2 (cOViD-19) treatment, Drug Resist Updat, doi:10.1016/j.drup.2021.100794
Goldman, association of remdesivir treatment with mortality among hospitalized adults with cOViD-19 in the United states, JaMa Netw Open, doi:10.1001/jamanetworkopen.2022.44505
Guzmán-Morales, Ledesma-Colunga, Mg, Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis, J clin invest, doi:10.1172/JCI69485
Je, Lara-Zárate L, Ochoa-Zarzosa A, effects of prolactin on innate immunity of infectious diseases, Open Neuroendocrinol J
Ka, Hall, Clark Rs, Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure, J immunol, doi:10.4049/jimmunol.174.6.3765
Martinez, Gil C, Gastaminza, Domperidone for use as antiviral agent, eP
Matalka, Prolactin enhances production of interferon-gamma, interleukin-12, and interleukin-10, but not of tumor necrosis factor-alpha, in a stimulusspecific manner, cytokine, doi:10.1016/s1043-4666(02)00496-9
Mbaeyi Sa, Walensky, cDc interim recommendations for fully vaccinated people: an important first step, JaMa, doi:10.1001/jama.2021.4367
Me, Prolactin and autoimmune diseases in humans, acta Biomed
Mirabelli C, Wotring, Zhang Cj, Morphological cell profiling of saRs-coV-2 infection identifies drug repurposing candidates for cOViD-19, Proc Natl acad sci U s a, doi:10.1073/pnas.2105815118
Ochoa-Amaya Je, Marino, attenuated allergic inflammatory response in the lungs during lactation, life sci, doi:10.1016/j.lfs.2016.03.027
Ochoa-Amaya Je, short-term hyperprolactinemia decreases allergic inflammatory response of the lungs, life sci, doi:10.1016/j.lfs.2015.10.016
Orlander H, Jarvis, imipramine induced elevation of prolactin levels in patients with hiV/aiDs improved their immune status, West indian Med J
Parra A, Ramírez-Peredo, Moderate hyperprolactinemia is associated with survival in patients with acute graft-versus-host disease after allogeneic stem cell transplantation, hematology, doi:10.1179/102453312X13221316477930
Pereira-Suarez Al, López-Rincón, Pam, Prolactin in inflammatory response, adv exp Med Biol, doi:10.1007/978-3-319-12114-7_11
Peña, immunostimulatory effect of salmon prolactin on expression of toll-like receptors in Oncorhynchus mykiss infected with Piscirickettsia salmonis, Fish Physiol Biochem, doi:10.1007/s10695-015-0155-5
Rivasi, Bulgaresi, Mossello, course and lethality of saRs-coV-2 epidemic in nursing homes after vaccination in Florence, italy, Vaccines, doi:10.3390/vaccines9101174
Shelly S, Boaz, Orbach, Prolactin and autoimmunity, autoimmun Rev, doi:10.1016/j.autrev.2011.11.009
Sykes L, Da, Yap, changes in the th1:th2 cytokine bias in pregnancy and the effects of the anti-inflammatory cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2, Mediators inflamm, doi:10.1155/2012/416739
Vila Méndez Ml, García Ar, efficacy of bromhexine versus standard of care in reducing viral load in patients with mild-to-moderate cOViD-19 disease attended in primary care: a randomized open-label trial, J clin Med, doi:10.3390/jcm12010142
Wen, efficacy and safety or three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for cOViD-19: a meta-analysis, ann Med, doi:10.1080/0785389.2022.2034936
Wu, Liu, Guo, Prolactin inhibits the progression of intervertebral disc degeneration through inactivation of the NF-κB pathway in rats, cell Death Dis, doi:10.1038/s41419-017-0151-z
Zellweger, Wichmann, Metoclopramide: a novel and safe immunomodulating agent for restoring the depressed macrophage immune function after hemorrhage, J trauma, doi:10.1097/00005373-199801000-00006
DOI record:
{
"DOI": "10.1080/07853890.2023.2268535",
"ISSN": [
"0785-3890",
"1365-2060"
],
"URL": "http://dx.doi.org/10.1080/07853890.2023.2268535",
"alternative-id": [
"10.1080/07853890.2023.2268535"
],
"article-number": "2268535",
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=iann20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=iann20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-03-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-10-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-10-17"
}
],
"author": [
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Rabanal Basalo",
"given": "Alejandro",
"sequence": "first"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Navarro Pablos",
"given": "Mercedes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Fronteras, Torrejón de Ardoz, Spain"
}
],
"family": "Viejo Pinero",
"given": "Nuria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Fronteras, Torrejón de Ardoz, Spain"
}
],
"family": "Vila Méndez",
"given": "María Luz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Fronteras, Torrejón de Ardoz, Spain"
}
],
"family": "Molina Barcena",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Benita de Ávila, Madrid, Spain"
}
],
"family": "Montilla Bernabé",
"given": "Aránzazu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Benita de Ávila, Madrid, Spain"
}
],
"family": "Villanueva Morán",
"given": "María del Pilar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Benita de Ávila, Madrid, Spain"
}
],
"family": "Blanco Gallego",
"given": "Ana María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Benita de Ávila, Madrid, Spain"
}
],
"family": "Guirao Sánchez",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Benita de Ávila, Madrid, Spain"
}
],
"family": "Juárez Antón",
"given": "Salvador",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Benita de Ávila, Madrid, Spain"
}
],
"family": "Fernández Rodríguez",
"given": "Ángela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Baviera, Madrid, Spain"
}
],
"family": "Revuelta Puigdollers",
"given": "María Luisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Baviera, Madrid, Spain"
}
],
"family": "Sarriá Sánchez",
"given": "María Teresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Baviera, Madrid, Spain"
}
],
"family": "Martín Alegre",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Alperchines, San Fernando de Henares, Spain"
}
],
"family": "Martínez Álvarez",
"given": "Miguel Ángel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Alperchines, San Fernando de Henares, Spain"
}
],
"family": "Mestre de Juan",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Mielgo Salvador",
"given": "Rebeca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Gijón Seco",
"given": "María Teresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. General Fanjul, Madrid, Spain"
}
],
"family": "Saníger Herrera",
"given": "José Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. General Fanjul, Madrid, Spain"
}
],
"family": "Rodríguez Jiménez",
"given": "María Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Navas de la Peña",
"given": "Begoña",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Daroca, Madrid, Spain"
}
],
"family": "Santa Cruz Hernández",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Abad Esteban",
"given": "Ana María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "Díaz Martín",
"given": "Rebeca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Los Yébenes, Madrid, Spain"
}
],
"family": "García Pérez",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Reina Victoria, Madrid, Spain"
}
],
"family": "Herrero Vanrell",
"given": "Paloma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Reina Victoria, Madrid, Spain"
}
],
"family": "Arias de Saavedra Criado",
"given": "María Isabel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Reina Victoria, Madrid, Spain"
}
],
"family": "Vaquero Vinent",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Reina Victoria, Madrid, Spain"
}
],
"family": "López Gómez",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Monmar, Las Rozas, Spain"
}
],
"family": "Montegrifo Rentero",
"given": "Víctor Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Monmar, Las Rozas, Spain"
}
],
"family": "Simón Miguel",
"given": "Lucía",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Las Rozas El Abajón, Las Rozas, Spain"
}
],
"family": "Campo Martos",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Las Rozas El Abajón, Las Rozas, Spain"
}
],
"family": "Ortiz Zamorano",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Villablanca, Madrid, Spain"
}
],
"family": "Izquierdo Zamarriego",
"given": "María Jesús",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Daroca, Madrid, Spain"
}
],
"family": "Vázquez Carrión",
"given": "Izíar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "C.S. Dos de Mayo, Móstoles, Spain"
}
],
"family": "López Valero",
"given": "Rosa María",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3882-6081",
"affiliation": [
{
"name": "Centro de Investigaciones Biológicas ‘Margarita Salas’, CSIC, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Gil",
"given": "Carmen",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2707-8110",
"affiliation": [
{
"name": "Centro de Investigaciones Biológicas ‘Margarita Salas’, CSIC, Madrid, Spain"
},
{
"name": "Centro de Investigación Biomédica en Red en Enfermedades Neurodegenerativas, ISCiii, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Martínez",
"given": "Ana",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5853-2307",
"affiliation": [
{
"name": "Medical Department, E-C-BIO, S.L., Las Rozas, Spain"
}
],
"authenticated-orcid": false,
"family": "Soler López",
"given": "Begoña",
"sequence": "additional"
}
],
"container-title": "Annals of Medicine",
"container-title-short": "Annals of Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2023,
10,
17
]
],
"date-time": "2023-10-17T22:07:17Z",
"timestamp": 1697580437000
},
"deposited": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T12:08:32Z",
"timestamp": 1708430912000
},
"funder": [
{
"award": [
"PIE 201980E024"
],
"name": "CSIC"
},
{
"award": [
"2020/2094"
],
"name": "European Commission: NextGeneration EU"
},
{
"name": "Agencia Estatal Consejo Superior de Investigaciones Científicas, M.P."
}
],
"indexed": {
"date-parts": [
[
2025,
7,
30
]
],
"date-time": "2025-07-30T11:31:30Z",
"timestamp": 1753875090953,
"version": "3.41.2"
},
"is-referenced-by-count": 2,
"issue": "2",
"issued": {
"date-parts": [
[
2023,
10,
17
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2023,
12,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
17
]
],
"date-time": "2023-10-17T00:00:00Z",
"timestamp": 1697500800000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/07853890.2023.2268535",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2023,
10,
17
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
17
]
]
},
"published-print": {
"date-parts": [
[
2023,
12,
12
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1073/pnas.2105815118",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_2_1"
},
{
"key": "e_1_3_5_3_1",
"unstructured": "Martinez A Gil C Gastaminza P et al. Domperidone for use as antiviral agent. EP3949965 WO2022028839 priority date 07/08/2020."
},
{
"DOI": "10.1016/j.mehy.2020.110208",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_4_1"
},
{
"DOI": "10.1007/s11102-005-5082-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_5_1"
},
{
"DOI": "10.3181/00379727-215-44111",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_6_1"
},
{
"DOI": "10.3389/fimmu.2018.00073",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_7_1"
},
{
"DOI": "10.1007/978-3-319-12114-7_11",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_8_1"
},
{
"DOI": "10.1155/2012/416739",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_9_1"
},
{
"DOI": "10.1179/102453312X13221316477930",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_10_1"
},
{
"DOI": "10.1210/rp.57.1.435",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_11_1"
},
{
"DOI": "10.1016/s1043-4666(02)00496-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_12_1"
},
{
"article-title": "Prolactin and autoimmune diseases in humans",
"author": "Chuang E",
"first-page": "255",
"issue": "1",
"journal-title": "Acta Biomed",
"key": "e_1_3_5_13_1",
"unstructured": "Chuang E, Molitch ME. Prolactin and autoimmune diseases in humans. Acta Biomed. 2007;78(Suppl 1):255–261.",
"volume": "78",
"year": "2007"
},
{
"DOI": "10.1172/JCI69485",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_14_1"
},
{
"article-title": "The role of neuroendocrine system in the pathogenesis of rheumatic disease (minireview)",
"author": "Imrich R.",
"first-page": "95",
"journal-title": "Endocr Regul",
"key": "e_1_3_5_15_1",
"unstructured": "Imrich R. The role of neuroendocrine system in the pathogenesis of rheumatic disease (minireview). Endocr Regul. 2002;36:95–106.",
"volume": "36",
"year": "2002"
},
{
"DOI": "10.1016/j.autrev.2011.11.009",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_16_1"
},
{
"DOI": "10.1136/adc.65.9.977",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_17_1"
},
{
"DOI": "10.1097/00005373-199801000-00006",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_18_1"
},
{
"article-title": "Imipramine induced elevation of prolactin levels in patients with HIV/AIDS improved their immune status",
"author": "Orlander H",
"first-page": "207",
"issue": "3",
"journal-title": "West Indian Med J",
"key": "e_1_3_5_19_1",
"unstructured": "Orlander H, Peter S, Jarvis M, et al. Imipramine induced elevation of prolactin levels in patients with HIV/AIDS improved their immune status. West Indian Med J. 2009;58(3):207–213.",
"volume": "58",
"year": "2009"
},
{
"DOI": "10.1038/s41419-017-0151-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_20_1"
},
{
"DOI": "10.1016/j.lfs.2015.10.016",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_21_1"
},
{
"DOI": "10.1016/j.lfs.2016.03.027",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_22_1"
},
{
"article-title": "Effects of prolactin on innate immunity of infectious diseases",
"author": "López-Mesa JE",
"first-page": "175",
"journal-title": "Open Neuroendocrinol J",
"key": "e_1_3_5_23_1",
"unstructured": "López-Mesa JE, Lara-Zárate L, Ochoa-Zarzosa A. Effects of prolactin on innate immunity of infectious diseases. Open Neuroendocrinol J. 2010;3:175–179.",
"volume": "3",
"year": "2010"
},
{
"DOI": "10.1007/s10695-015-0155-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_24_1"
},
{
"DOI": "10.33549/physiolres.932262",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_25_1"
},
{
"DOI": "10.4049/jimmunol.174.6.3765",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_26_1"
},
{
"key": "e_1_3_5_27_1",
"unstructured": "Sociedad Española de Médicos de Atención Primaria (SEMERGEN). Protocolo de Actuación en Pacientes con COVID-19 Asistidos en Atención Primaria. Madrid Spain: Sociedad Española de Médicos de Atención Primaria (SEMERGEN); 2020. ISBN 978-84-16269-52-5. [cited 2022 Nov 22]. Available from: https://www.semergen.es/files/docs/COVID-19/Documentos/ferrer-covid-19.pdf"
},
{
"key": "e_1_3_5_28_1",
"unstructured": "World Health Organization. COVID-19 clinical management. This document is the update of an interim guidance originally published under the Title “Clinical management of COVID-19: interim guidance 27 May 2020”. World Health Organization; 2021. (WHO reference number: WHO/2019-nCoV/clinical/2021.1). [cited 2022 Nov 22]. Available from: https://www.semergen.es/files/docs/COVID-19/Documentos/who-guide-covig192021.1-eng.pdf"
},
{
"DOI": "10.3390/vaccines9101174",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_29_1"
},
{
"DOI": "10.1001/jama.2021.4367",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_30_1"
},
{
"key": "e_1_3_5_31_1",
"unstructured": "Actualización de la Situación Epidemiológica de las Variantes SARS-CoV-2 en España. Ministerio de Sanidad; 2022 Sep 26 [cited 2022 No 22]. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/ ccayes/alertasActual/nCov/documentos/COVID19_Actualizacion_variantes_20220926.pdf"
},
{
"DOI": "10.1001/jamanetworkopen.2022.44505",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_32_1"
},
{
"DOI": "10.1080/0785389.2022.2034936",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_33_1"
},
{
"DOI": "10.2147/DDDT.S354841",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_34_1"
},
{
"DOI": "10.1016/j.drup.2021.100794",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_35_1"
},
{
"DOI": "10.3390/jcm12010142",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_36_1"
},
{
"key": "e_1_3_5_37_1",
"unstructured": "Ministerio de Sanidad. Enfermedad por coronavirus COVID-19. Actualización 2020 Nov 12 [cited 2022 Nov 22]. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/ITCoronavirus/home.htm"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/07853890.2023.2268535"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A randomized, double-blind study on the efficacy of oral domperidone versus placebo for reducing SARS-CoV-2 viral load in mild-to-moderate COVID-19 patients in primary health care",
"type": "journal-article",
"update-policy": "https://doi.org/10.1080/tandf_crossmark_01",
"volume": "55"
}