Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics
et al., eBioMedicine, doi:10.1016/j.ebiom.2022.103856, DEFINE, NCT04473053, Feb 2022
RCT 42 hospitalized patients with COVID-19 pneumonitis showing no benefit with intravenous nafamostat mesylate.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers TMPRSS2 inhibitors and nafamostat.
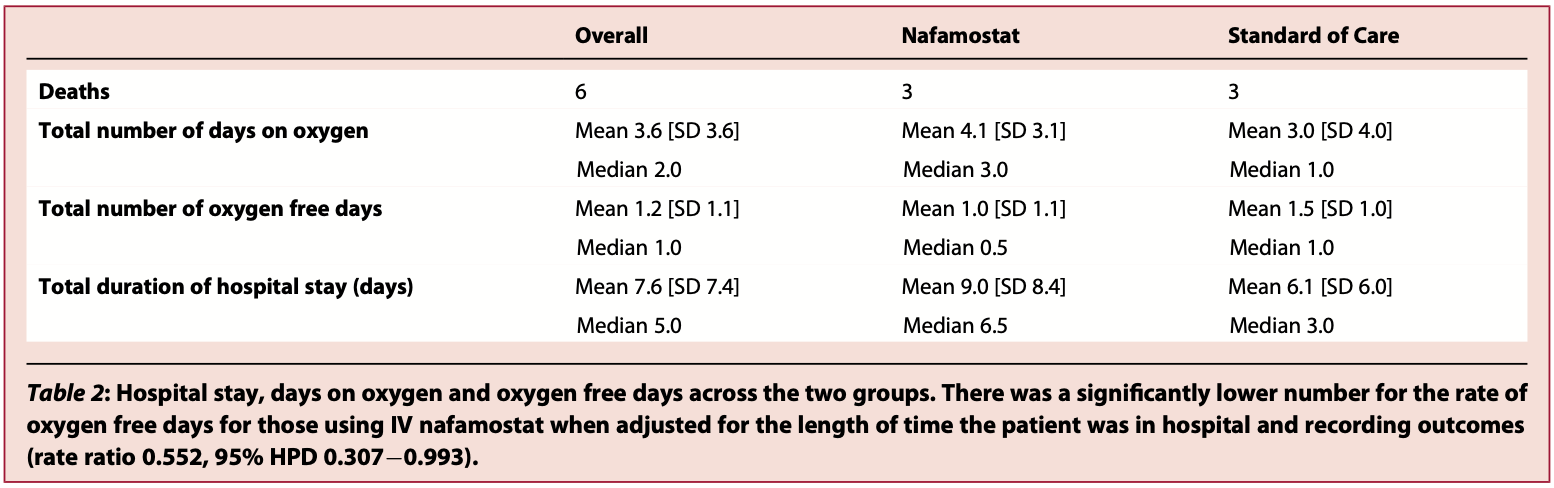
|
risk of death, no change, RR 1.00, p = 1.00, treatment 3 of 21 (14.3%), control 3 of 21 (14.3%).
|
|
hospitalization time, 47.5% higher, relative time 1.48, p = 0.21, treatment mean 9.0 (±8.4) n=21, control mean 6.1 (±6.0) n=21.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Quinn et al., 28 Feb 2022, Randomized Controlled Trial, United Kingdom, peer-reviewed, mean age 63.6, 49 authors, study period September 2020 - February 2021, average treatment delay 8.5 days, trial NCT04473053 (history) (DEFINE).
Contact: kev.dhaliwal@ed.ac.uk.
Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics
eBioMedicine, doi:10.1016/j.ebiom.2022.103856
Background Many repurposed drugs have progressed rapidly to Phase 2 and 3 trials in COVID19 without characterisation of Pharmacokinetics /Pharmacodynamics including safety data. One such drug is nafamostat mesylate. Methods We present the findings of a phase Ib/IIa open label, platform randomised controlled trial of intravenous nafamostat in hospitalised patients with confirmed COVID-19 pneumonitis. Patients were assigned randomly to standard of care (SoC), nafamostat or an alternative therapy. Nafamostat was administered as an intravenous infusion at a dose of 0.2 mg/kg/h for a maximum of seven days. The analysis population included those who received any dose of the trial drug and all patients randomised to SoC. The primary outcomes of our trial were the safety and tolerability of intravenous nafamostat as an add on therapy for patients hospitalised with COVID-19 pneumonitis.
preparation. TQ & EG are joint first authors and have verified the underlying data.
Declaration of interests The authors report no conflict of interests.
Supplementary materials Supplementary material associated with this article can be found in the online version at doi:10.1016/j. ebiom.2022.103856.
References
Abani, Abbas, Abbas, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, The Lancet
Aoyama, Ino, Ozeki, Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments, Jpn J Pharmacol
Cao, Zhang, Yu, Shao, Chen et al., A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: more accurate evaluation for pharmacokinetic study, Anal Bioanal Chem
Choi, Kang, Jang, Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial, Medicine (Baltimore)
Cui, Chen, Li, Liu, Wang, Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia, Journal of Thrombosis and Haemostasis
Di, The impact of carboxylesterases in drug metabolism and pharmacokinetics, Curr Drug Metab
Doi, Ikeda, Hayase, Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series, Critical Care
Fujii, Hitomi, New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin, Biochimica et Biophysica Acta (BBA) -Enzymology
Gaughan, Quinn, Bruce, DEFINE: a phase iia randomised controlled trial to evaluate repurposed treatments for COVID-19, medRxiv
Groene, Sappel, Saller, Functional testing of tranexamic acid effects in patients undergoing elective orthopaedic surgery, J Thromb Thrombolysis
Group, Dexamethasone in hospitalized patients with Covid-19, New England Journal of Medicine
Hoffmann, Schroeder, Kleine-Weber, Müller, Drosten et al., Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19, Antimicrobial Agents and Chemotherapy
Jang, Rhee, Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy, Int J Infect Dis
Kammerer, Groene, Sappel, Functional testing for tranexamic acid duration of action using modified viscoelastometry, Transfusion Medicine and Hemotherapy
Kirkpatrick, Millard, Evaluation of nafamostat mesylate safety and inhibition of SARS-CoV-2 replication using a 3-dimensional human airway epithelia model, bioRxiv
Ko, Jeon, Ryu, Kim, Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells, Journal of Medical Virology
Kuri-Cervantes, Pampena, Meng, Comprehensive mapping of immune perturbations associated with severe COVID-19, Science Immunology
Levi, Thachil, Iba, Levy, Coagulation abnormalities and thrombosis in patients with COVID-19, Lancet Haematol
Li, Meyerholz, Bartlett, Mccray, The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 pulmonary infection in mouse models of COVID-19
Malato, Dentali, Siragusa, The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes, Blood Transfus
Mathew, Giles, Baxter, Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications, Science
Mccullagh, Routledge, Generalized Linear Models, doi:10.1201/9780203753736
Muto, Imai, Asano, Mechanisms of hyperkalemia caused by nafamostat mesilate, Gen Pharmacol
Okajima, Uchiba, Murakami, Nafamostat, Cardiovascular Drug Reviews
Organization, Novel Coronavirus -China
Shankar-Hari, Vale, Godolphin, Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis, Jama
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, Journal of Thrombosis and Haemostasis
Tldi, COVID-19 Drug interactions
Whiting, Dinardo, TEG and ROTEM: Technology and clinical applications, American Journal of Hematology
Yamamoto, Kiso, Sakai-Tagawa, The anticoagulant nafamostat potently inhibits sars-cov-2 s protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a celltype-dependent manner, Viruses
Yamamoto, Matsuyama, Li, Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus s protein-mediated membrane fusion using the splitprotein-based cell-cell fusion assay, Antimicrob Agents Chemother
Yamaori, Fujiyama, Kushihara, Involvement of human blood arylesterases and liver microsomal carboxylesterases in nafamostat hydrolysis, Drug Metabolism and Pharmacokinetics
Yuriditsky, Horowitz, Merchan, Thromboelastography profiles of critically Ill patients with coronavirus disease 2019, Crit Care Med
Zeng, Evans, King, SARS-CoV-2 Spreads through cellto-cell transmission, bioRxiv
DOI record:
{
"DOI": "10.1016/j.ebiom.2022.103856",
"ISSN": [
"2352-3964"
],
"URL": "http://dx.doi.org/10.1016/j.ebiom.2022.103856",
"alternative-id": [
"S2352396422000408"
],
"article-number": "103856",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eBioMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ebiom.2022.103856"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3544-4333",
"affiliation": [],
"authenticated-orcid": false,
"family": "Quinn",
"given": "Tom M.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9295-5503",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gaughan",
"given": "Erin E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruce",
"given": "Annya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Antonelli",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O'Connor",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McNamara",
"given": "Sarah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3454-2855",
"affiliation": [],
"authenticated-orcid": false,
"family": "Koch",
"given": "Oliver",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacKintosh",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dockrell",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walsh",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blyth",
"given": "Kevin G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Church",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schwarze",
"given": "Jürgen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boz",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valanciute",
"given": "Asta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burgess",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Emanuel",
"given": "Philip",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3209-9490",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mills",
"given": "Bethany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rinaldi",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hardisty",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mills",
"given": "Ross",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Findlay",
"given": "Emily Gwyer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jabbal",
"given": "Sunny",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7767-472X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Duncan",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plant",
"given": "Sinéad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marshall",
"given": "Adam D.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Young",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russell",
"given": "Kay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scholefield",
"given": "Emma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0576-3387",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nimmo",
"given": "Alastair F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nazarov",
"given": "Islom B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Churchill",
"given": "Grant C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4733-1205",
"affiliation": [],
"authenticated-orcid": false,
"family": "McCullagh",
"given": "James S.O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ebrahimi",
"given": "Kourosh H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrett",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Templeton",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rannard",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Finlayson",
"given": "Keith",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5338-2538",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shankar-Hari",
"given": "Manu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norrie",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parker",
"given": "Richard A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4605-1682",
"affiliation": [],
"authenticated-orcid": false,
"family": "Akram",
"given": "Ahsan R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1380-6655",
"affiliation": [],
"authenticated-orcid": false,
"family": "Anthony",
"given": "Daniel C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dear",
"given": "James W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirani",
"given": "Nik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhaliwal",
"given": "Kevin",
"sequence": "additional"
}
],
"container-title": "eBioMedicine",
"container-title-short": "eBioMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T23:12:05Z",
"timestamp": 1644534725000
},
"deposited": {
"date-parts": [
[
2022,
7,
22
]
],
"date-time": "2022-07-22T07:37:12Z",
"timestamp": 1658475432000
},
"indexed": {
"date-parts": [
[
2025,
4,
26
]
],
"date-time": "2025-04-26T13:06:17Z",
"timestamp": 1745672777130
},
"is-referenced-by-count": 44,
"issued": {
"date-parts": [
[
2022,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
18
]
],
"date-time": "2022-01-18T00:00:00Z",
"timestamp": 1642464000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2352396422000408?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2352396422000408?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "103856",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.ebiom.2022.103856_bib0001",
"series-title": "Novel Coronavirus – China",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.11330",
"article-title": "Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis",
"author": "Shankar-Hari",
"doi-asserted-by": "crossref",
"first-page": "499",
"issue": "6",
"journal-title": "Jama",
"key": "10.1016/j.ebiom.2022.103856_bib0002",
"volume": "326",
"year": "2021"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Group",
"first-page": "693",
"issue": "8",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.ebiom.2022.103856_bib0003",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Abani",
"doi-asserted-by": "crossref",
"first-page": "1637",
"issue": "10285",
"journal-title": "The Lancet",
"key": "10.1016/j.ebiom.2022.103856_bib0004",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1128/AAC.01043-16",
"article-title": "Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus s protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "6532",
"issue": "11",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ebiom.2022.103856_bib0005",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.3390/v12060629",
"article-title": "The anticoagulant nafamostat potently inhibits sars-cov-2 s protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "629",
"issue": "6",
"journal-title": "Viruses",
"key": "10.1016/j.ebiom.2022.103856_bib0006",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00754-20",
"article-title": "Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "e00754",
"issue": "6",
"journal-title": "Antimicrobial Agents and Chemotherapy",
"key": "10.1016/j.ebiom.2022.103856_bib0007",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1128/mBio.00970-21",
"article-title": "The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 pulmonary infection in mouse models of COVID-19",
"author": "Li",
"doi-asserted-by": "crossref",
"journal-title": "mBio",
"key": "10.1016/j.ebiom.2022.103856_bib0008",
"year": "2021"
},
{
"DOI": "10.1097/MD.0000000000002392",
"article-title": "Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "e2392",
"issue": "52",
"journal-title": "Medicine (Baltimore)",
"key": "10.1016/j.ebiom.2022.103856_bib0009",
"volume": "94",
"year": "2015"
},
{
"DOI": "10.1111/j.1527-3466.1995.tb00213.x",
"author": "Okajima",
"doi-asserted-by": "crossref",
"first-page": "51",
"issue": "1",
"journal-title": "Cardiovascular Drug Reviews",
"key": "10.1016/j.ebiom.2022.103856_bib0010",
"volume": "13",
"year": "1995"
},
{
"DOI": "10.1254/jjp.35.203",
"article-title": "Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments",
"author": "Aoyama",
"doi-asserted-by": "crossref",
"first-page": "203",
"issue": "3",
"journal-title": "Jpn J Pharmacol",
"key": "10.1016/j.ebiom.2022.103856_bib0011",
"volume": "35",
"year": "1984"
},
{
"DOI": "10.1016/0005-2744(81)90023-1",
"article-title": "New synthetic inhibitors of C1r̄, C1 esterase, thrombin, plasmin, kallikrein and trypsin",
"author": "Fujii",
"doi-asserted-by": "crossref",
"first-page": "342",
"issue": "2",
"journal-title": "Biochimica et Biophysica Acta (BBA) - Enzymology",
"key": "10.1016/j.ebiom.2022.103856_bib0012",
"volume": "661",
"year": "1981"
},
{
"article-title": "DEFINE: a phase iia randomised controlled trial to evaluate repurposed treatments for COVID-19",
"author": "Gaughan",
"journal-title": "medRxiv",
"key": "10.1016/j.ebiom.2022.103856_bib0013",
"year": "2021"
},
{
"DOI": "10.1002/ajh.23599",
"article-title": "TEG and ROTEM: Technology and clinical applications",
"author": "Whiting",
"doi-asserted-by": "crossref",
"first-page": "228",
"issue": "2",
"journal-title": "American Journal of Hematology",
"key": "10.1016/j.ebiom.2022.103856_bib0014",
"volume": "89",
"year": "2014"
},
{
"DOI": "10.1007/s11239-020-02272-8",
"article-title": "Functional testing of tranexamic acid effects in patients undergoing elective orthopaedic surgery",
"author": "Groene",
"doi-asserted-by": "crossref",
"first-page": "989",
"issue": "4",
"journal-title": "J Thromb Thrombolysis",
"key": "10.1016/j.ebiom.2022.103856_bib0015",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1159/000511230",
"article-title": "Functional testing for tranexamic acid duration of action using modified viscoelastometry",
"author": "Kammerer",
"doi-asserted-by": "crossref",
"first-page": "109",
"issue": "2",
"journal-title": "Transfusion Medicine and Hemotherapy",
"key": "10.1016/j.ebiom.2022.103856_bib0016",
"volume": "48",
"year": "2021"
},
{
"author": "McCullagh",
"key": "10.1016/j.ebiom.2022.103856_bib0017",
"series-title": "Generalized Linear Models",
"year": "1983"
},
{
"DOI": "10.1002/jmv.26397",
"article-title": "Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "1403",
"issue": "3",
"journal-title": "Journal of Medical Virology",
"key": "10.1016/j.ebiom.2022.103856_bib0018",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.05.072",
"article-title": "Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ebiom.2022.103856_bib0019",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03078-z",
"article-title": "Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "392",
"issue": "1",
"journal-title": "Critical Care",
"key": "10.1016/j.ebiom.2022.103856_bib0020",
"volume": "24",
"year": "2020"
},
{
"key": "10.1016/j.ebiom.2022.103856_bib0021",
"unstructured": "EUnetHTA Joint Action 3 WP4: NAFAMOSTAT FOR THE TREATMENT OF COVID-19. Version 7.0, May 2021"
},
{
"DOI": "10.1016/0306-3623(95)00072-0",
"article-title": "Mechanisms of hyperkalemia caused by nafamostat mesilate",
"author": "Muto",
"doi-asserted-by": "crossref",
"first-page": "1627",
"issue": "8",
"journal-title": "Gen Pharmacol",
"key": "10.1016/j.ebiom.2022.103856_bib0022",
"volume": "26",
"year": "1995"
},
{
"DOI": "10.1007/s00216-008-2054-4",
"article-title": "A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: more accurate evaluation for pharmacokinetic study",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1063",
"issue": "3",
"journal-title": "Anal Bioanal Chem",
"key": "10.1016/j.ebiom.2022.103856_bib0023",
"volume": "391",
"year": "2008"
},
{
"DOI": "10.2133/dmpk.21.147",
"article-title": "Involvement of human blood arylesterases and liver microsomal carboxylesterases in nafamostat hydrolysis",
"author": "Yamaori",
"doi-asserted-by": "crossref",
"first-page": "147",
"issue": "2",
"journal-title": "Drug Metabolism and Pharmacokinetics",
"key": "10.1016/j.ebiom.2022.103856_bib0024",
"volume": "21",
"year": "2006"
},
{
"DOI": "10.2174/1389200219666180821094502",
"article-title": "The impact of carboxylesterases in drug metabolism and pharmacokinetics",
"author": "Di",
"doi-asserted-by": "crossref",
"first-page": "91",
"issue": "2",
"journal-title": "Curr Drug Metab",
"key": "10.1016/j.ebiom.2022.103856_bib0025",
"volume": "20",
"year": "2019"
},
{
"key": "10.1016/j.ebiom.2022.103856_bib0026",
"unstructured": "group TLdi. COVID-19 Drug interactions, 2021 [accessed 5/8/21.2021]."
},
{
"DOI": "10.1111/jth.14830",
"article-title": "Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia",
"author": "Cui",
"doi-asserted-by": "crossref",
"first-page": "1421",
"issue": "6",
"journal-title": "Journal of Thrombosis and Haemostasis",
"key": "10.1016/j.ebiom.2022.103856_bib0027",
"volume": "18",
"year": "2020"
},
{
"article-title": "The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes",
"author": "Malato",
"first-page": "559",
"issue": "4",
"journal-title": "Blood Transfus",
"key": "10.1016/j.ebiom.2022.103856_bib0028",
"volume": "13",
"year": "2015"
},
{
"DOI": "10.1111/jth.14768",
"article-title": "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "844",
"issue": "4",
"journal-title": "Journal of Thrombosis and Haemostasis",
"key": "10.1016/j.ebiom.2022.103856_bib0029",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30145-9",
"article-title": "Coagulation abnormalities and thrombosis in patients with COVID-19",
"author": "Levi",
"doi-asserted-by": "crossref",
"first-page": "e438",
"issue": "6",
"journal-title": "Lancet Haematol",
"key": "10.1016/j.ebiom.2022.103856_bib0030",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000004471",
"article-title": "Thromboelastography profiles of critically Ill patients with coronavirus disease 2019",
"author": "Yuriditsky",
"doi-asserted-by": "crossref",
"first-page": "1319",
"issue": "9",
"journal-title": "Crit Care Med",
"key": "10.1016/j.ebiom.2022.103856_bib0031",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1126/science.abc8511",
"article-title": "Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications",
"author": "Mathew",
"doi-asserted-by": "crossref",
"first-page": "eabc8511",
"issue": "6508",
"journal-title": "Science",
"key": "10.1016/j.ebiom.2022.103856_bib0032",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.abd7114",
"article-title": "Comprehensive mapping of immune perturbations associated with severe COVID-19",
"author": "Kuri-Cervantes",
"doi-asserted-by": "crossref",
"first-page": "eabd7114",
"issue": "49",
"journal-title": "Science Immunology",
"key": "10.1016/j.ebiom.2022.103856_bib0033",
"volume": "5",
"year": "2020"
},
{
"article-title": "Evaluation of nafamostat mesylate safety and inhibition of SARS-CoV-2 replication using a 3-dimensional human airway epithelia model",
"author": "Kirkpatrick",
"journal-title": "bioRxiv",
"key": "10.1016/j.ebiom.2022.103856_bib0034",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 Spreads through cell-to-cell transmission",
"author": "Zeng",
"journal-title": "bioRxiv",
"key": "10.1016/j.ebiom.2022.103856_bib0035",
"year": "2021"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.10.06.21264648",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2352396422000408"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "76"
}
quinn