Efficacy of Favipiravir in the Treatment of Mild to Moderate COVID-19 Patients in Erbil: A Controlled Clinical Trial
et al., International Journal of Applied Sciences: Current and Future Research Trends, 13:1, May 2022
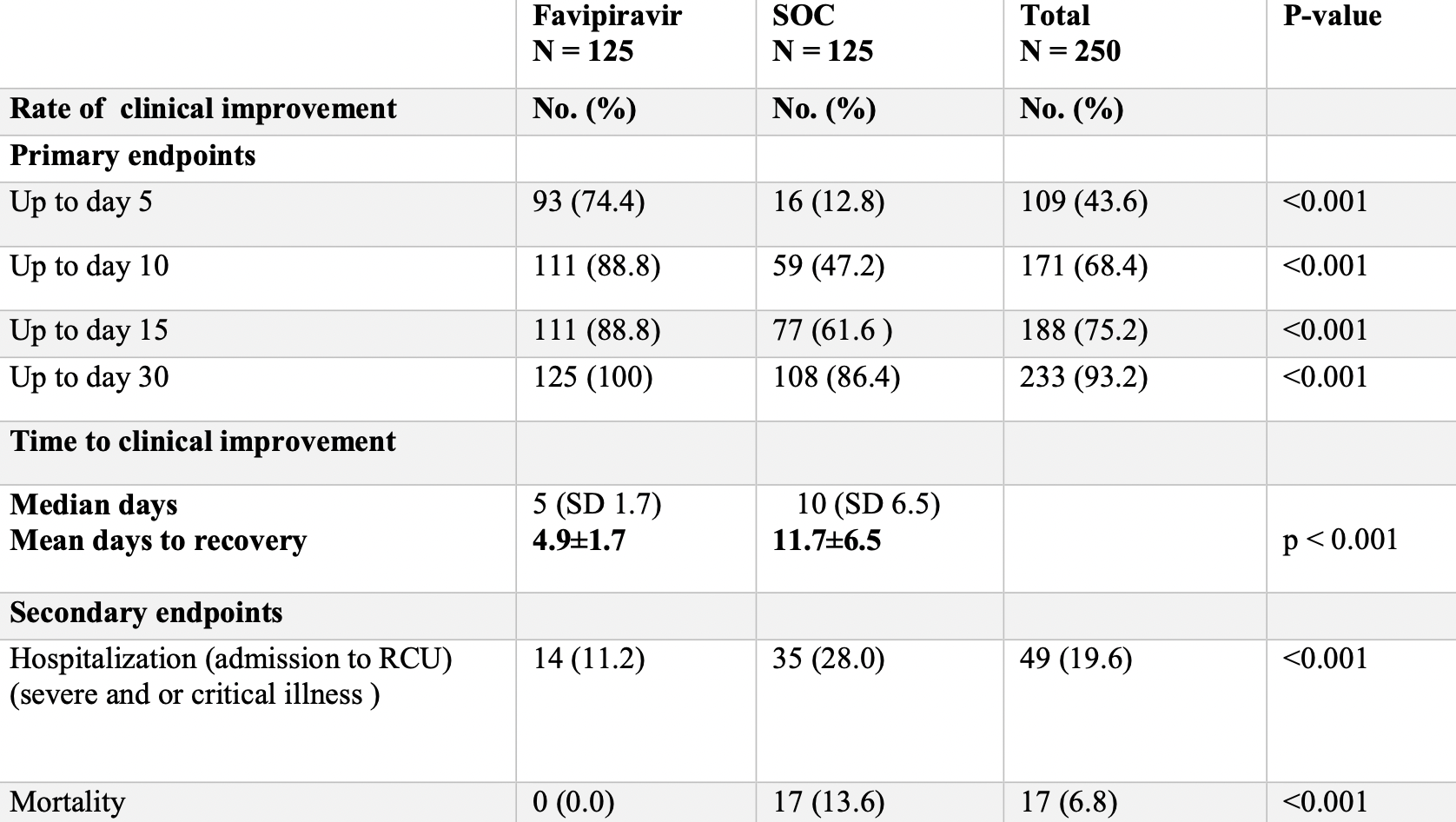
Prospective study with 125 favipiravir patients and 125 patients declining favipiravir treatment, showing lower mortality and improved recovery with treatment. All patients received vitamin C, D, and zinc. Favipiravir 3200mg day 1, followed by 600mg bid days 2-10.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 97.1% lower, RR 0.03, p < 0.001, treatment 0 of 125 (0.0%), control 17 of 125 (13.6%), NNT 7.4, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 30.
|
|
risk of hospitalization, 60.0% lower, RR 0.40, p = 0.001, treatment 14 of 125 (11.2%), control 35 of 125 (28.0%), NNT 6.0.
|
|
risk of no recovery, 97.1% lower, RR 0.03, p < 0.001, treatment 0 of 125 (0.0%), control 17 of 125 (13.6%), NNT 7.4, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 30, primary outcome.
|
|
risk of no recovery, 70.8% lower, RR 0.29, p < 0.001, treatment 14 of 125 (11.2%), control 48 of 125 (38.4%), NNT 3.7, day 15.
|
|
risk of no recovery, 78.8% lower, RR 0.21, p < 0.001, treatment 14 of 125 (11.2%), control 66 of 125 (52.8%), NNT 2.4, day 10.
|
|
risk of no recovery, 70.6% lower, RR 0.29, p < 0.001, treatment 32 of 125 (25.6%), control 109 of 125 (87.2%), NNT 1.6, day 5.
|
|
recovery time, 58.1% lower, relative time 0.42, p < 0.001, treatment 125, control 125.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Qadir et al., 23 May 2022, prospective, Iraq, peer-reviewed, 3 authors, study period 22 June, 2020 - 25 October, 2021.
International Journal of Applied Sciences: Current and Future Research Trends
Background and objectives: Favipiravir (FAV) is considered to have potential efficacy against the SARS-CoV-2 virus. We aimed to explore the efficacy of favipiravir in the treatment of mild and moderate cases of COVID-19 pneumonia. Methods: 250 patients of mild and moderate COVID-19 patients confirmed by reverse transcription-polymerase chain reaction (RT-PCR) were included from 22 nd of June 2020 till 25 th of October 2021, aged 18 to 90 years, 125 patients received FAV 3200 mg no day 1 followed by 600 mg twice daily (from day 2 -day 10). In another group, 125 patients did not receive favipiravir (SOC, standard of care group). They received paracetamol, vitamins D, and C plus Zinc, and azithromycin within the first 10 days of symptoms' onset. In both groups, the patients were monitored for clinical recovery on the 5 th ,10 th, 15 th days and after one month of receiving the therapeutic trials. Patients were enrolled from Rizgari Teaching Hospital, and from an outpatient respiratory private clinic. Both arms were comparable as regards demographic characteristics, severity, and comorbidities. It was a non-randomized -controlled trial. Results: On day five, the rate of clinical improvement in the FAV group (74.4%) was significantly (p < 0.001) higher than the rate in the SOC group (12.8%). On day 10, the mentioned rate was 88.8% in the FAV group compared with 47.2% in the SOC group (p < 0.001). The median time of clinical recovery was 6.5 days in the FAV group vs.
References
Abdulah, Qazli, Suleman, Response of the Public to Preventive Measures of COVID-19 in Iraqi Kurdistan, Disaster Med Public Health Prep, doi:10.1017/dmp.2020.233
Bhagat, Vora, Daxini, Dadhich, Patil et al., Clinical usefulness of favipiravir in moderate COVID-19 patients: Indian real-world experience, Indian Journal of Critical Care Medicine
Cai, Yang, Liu, Chen, Shu et al., treatment with favipiravir for COVID-19: an open-label control study, Experimental International Journal of Applied Sciences: Current and Future Research Trends (IJASCFRT)
Dabbous, El-Sayed, Assal, Elghazaly, Ebeid et al., Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial, Scientific reports
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proceedings of the Japan Academy, Series B
Hayden, Shindo, Influenza virus polymerase inhibitors in clinical development. Current opinion in infectious diseases
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The lancet
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial, medRxiv
Liu, Chang, Wang, Tsai, Hung et al., Prolonged virus shedding even after seroconversion in a patient with COVID-19, J Infect
Madelain, Oestereich, Graw, Nguyen, De Lamballerie et al., Ebola virus dynamics in mice treated with favipiravir, Antiviral research
Ruzhentsova, Oseshnyuk, Soluyanova, Dmitrikova, Mustafaev et al., Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19, American Journal of Translational Research
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, International Journal of Infectious Diseases
Zhonghua, He, Hu, Za, Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia, Bibl. Nac. Med