A Multicentre, Open Label, Randomised, Controlled, Basket, Pragmatic, Phase II, Clinical and Translational Study to Determine the Efficacy and Safety of Plitidepsin Versus Control in Immunocompromised Adult Patients With Symptomatic COVID-19 Requiring Hospital Care
, NCT05705167, NEREIDA, NCT05705167, Dec 2024
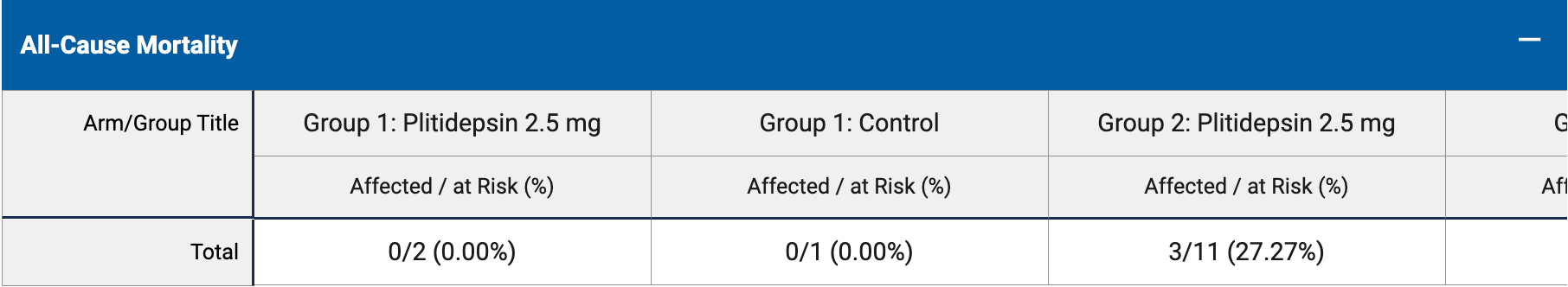
RCT 37 hospitalized immunocompromised patients, showing no significant benefit with plitidepsin treatment.
|
risk of death, 36.4% higher, RR 1.36, p = 1.00, treatment 5 of 22 (22.7%), control 1 of 6 (16.7%).
|
|
risk of death, 25.0% lower, RR 0.75, p = 1.00, treatment 3 of 20 (15.0%), control 1 of 5 (20.0%), NNT 20, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
PharmaMar et al., 2 Dec 2024, Randomized Controlled Trial, Belgium, preprint, 1 author, trial NCT05705167 (history) (NEREIDA).
Contact: clinicaltrials@pharmamar.com.