Mar 1 |
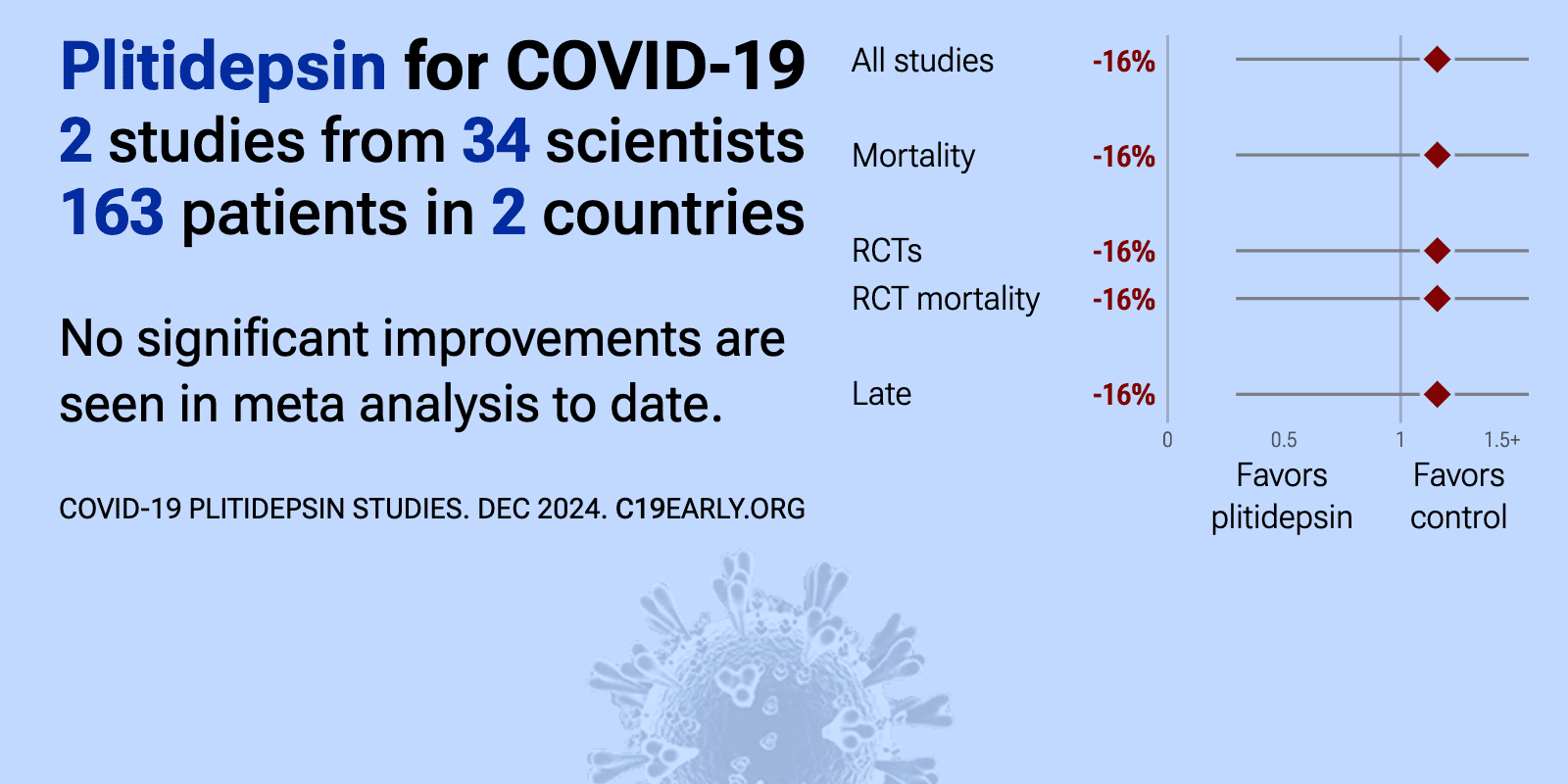
Meta-analysis of plitidepsin studies | |
| Meta-analysis of plitidepsin studies | ||
Dec 2 2024 |
, NCT05705167 | A Multicentre, Open Label, Randomised, Controlled, Basket, Pragmatic, Phase II, Clinical and Translational Study to Determine the Efficacy and Safety of Plitidepsin Versus Control in Immunocompromised Adult Patients With Symptomatic COVID-19 Requiring Hospital Care |
| 36% higher mortality (p=1). RCT 37 hospitalized immunocompromised patients, showing no significant benefit with plitidepsin treatment. | ||
Aug 26 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae227 | A Phase III Randomized Controlled Trial of Plitidepsin, a Marine-Derived Compound, in Hospitalized Adults With Moderate COVID-19 |
| 1% lower mortality (p=1). RCT with 205 hospitalized moderate COVID-19 patients showing a trend towards faster time to sustained withdrawal of oxygen supplementation with plitidepsin treatment (1.5 mg/day or 2.5 mg/day for 3 days) compared to standard of care. The .. | ||