A Phase III Randomized Controlled Trial of Plitidepsin, a Marine-Derived Compound, in Hospitalized Adults With Moderate COVID-19
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae227, NEPTUNO, NCT04784559, Aug 2024
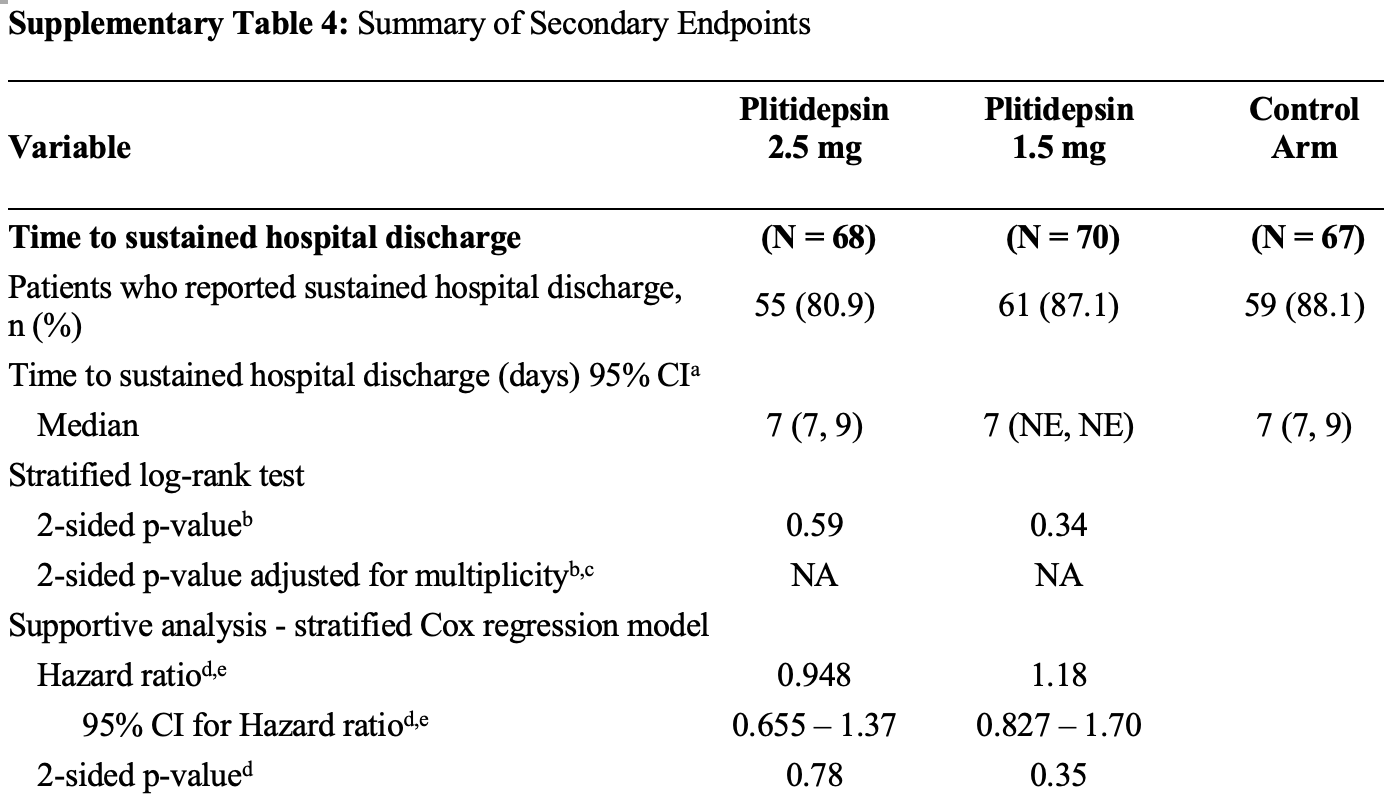
RCT with 205 hospitalized moderate COVID-19 patients showing a trend towards faster time to sustained withdrawal of oxygen supplementation with plitidepsin treatment (1.5 mg/day or 2.5 mg/day for 3 days) compared to standard of care. The trial was terminated early due to a drop in COVID-19-related hospitalizations. Plitidepsin was generally well tolerated.
|
risk of death, 1.5% lower, RR 0.99, p = 1.00, treatment 2 of 68 (2.9%), control 2 of 67 (3.0%), NNT 2278, 2.5mg.
|
|
risk of death, 52.1% lower, RR 0.48, p = 0.61, treatment 1 of 70 (1.4%), control 2 of 67 (3.0%), NNT 64, 1.5mg.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Landete et al., 26 Aug 2024, Randomized Controlled Trial, Spain, peer-reviewed, 33 authors, study period June 2021 - January 2023, trial NCT04784559 (history) (NEPTUNO).
Contact: jfvarona@hmhospitales.com, landete.pedro@gmail.com.
A Phase III Randomized Controlled Trial of Plitidepsin, a Marine-Derived Compound, in Hospitalized Adults With Moderate COVID-19
Clinical Infectious Diseases, doi:10.1093/cid/ciae227
Background. Plitidepsin has shown potent preclinical activity against severe acute respiratory syndrome coronavirus 2 and was generally well tolerated in a phase I trial of hospitalized patients with coronavirus disease 2019 . NEPTUNO, a phase III, multicenter, randomized, controlled trial, was designed to evaluate the efficacy and safety of plitidepsin in the management of moderate COVID-19 in hospitalized adult patients. Methods. Included patients had documented severe acute respiratory syndrome coronavirus 2 infection, required oxygen therapy, and had adequate organ function. The planned sample size was 609 patients. Patients were randomized 1:1:1 to at least 3 days of dexamethasone plus either plitidepsin (1.5 mg/day or 2.5 mg/day, for 3 days) or standard of care (control). The primary endpoint was the time to sustained withdrawal of supplemental oxygen. Secondary endpoints included time to sustained hospital discharge, clinical status, duration of oxygen support, percentage of patients requiring admission to the intensive care unit, and safety. Results. After randomizing 205 patients, NEPTUNO was discontinued due to a notable drop in COVID-19-related hospitalizations. Available data suggest a 2-day improvement in the median time to sustained oxygen therapy discontinuation (5 vs 7 days) favoring both plitidepsin arms (hazard ratio, 1.37; 95% confidence interval, .96-1.96; P = .08 for plitidepsin 1.5 mg vs control; hazard ratio, 1.06; 95% confidence interval, .73-1.53; P = .78 for plitidepsin 2.5 mg vs control). Plitidepsin was generally well tolerated.
Supplementary Data Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes Author contributions. O-A. C-S., L. P., M-C. L., A. K., P. V., S-P. I., P. G-V., M-C. F-A, E. M. L., E. P-A., J-M. C., V. E., C. H-T., G. P., M. T., J. F., P. G-O., A. L., M. M-M, M. M., R. P., M. T. P-R., D. R., P. R.: investigation, writing-review. P. L, J. F. V.: investigation, writing-review, validation. D. L-M.: supervision, project administration, and funding acquisition. E-M. S-L.: supervision, validation, and investigation. F. M.: supervision, validation. J. G.: software, methodology, formal analysis and writingreview, visualization. N. T.: software, data curation and validation. J. A. L-M: conceptualization, formal analysis, methodology and writing-original draft, review & editing, and visualization. J. J.: conceptualization, formal analysis, methodology and writing-original draft, review & editing, supervision, and funding acquisition. Acknowledgments. The authors thank the patients, and their relatives for their participation in this trial and also thank Timothy Silverstein for providing editorial support and Raquel Lloris for dedicated contributions to this trial. Data from this trial are not publicly available. Ethical Approval..
References
Aguareles, Fernandez, Outcomes and clinical characteristics of the compassionate use of plitidepsin for immunocompromised adult patients with COVID-19, Int J Infect Dis
Bouhaddou, Reuschl, Polacco, SARS-CoV-2 variants evolve convergent strategies to remodel the host response, Cell
Charlson, Szatrowski, Peterson, Gold, Validation of a combined comorbidity index, J Clin Epidemiol
Fall, Eldesouki, Sachithanandham, The displacement of the SARS-CoV-2 variant Delta with Omicron: an investigation of hospital admissions and upper respiratory viral loads, EBioMedicine
Losada, Munoz-Alonso, Garcia, Translation elongation factor eEF1A2 is a novel anticancer target for the marine natural product plitidepsin, Sci Rep
Mahoney, Barthel, Functional evaluation: the Barthel Index, Md State Med J
Rodon, Munoz-Basagoiti, Perez-Zsolt, Identification of plitidepsin as potent inhibitor of SARS-CoV-2-induced cytopathic effect after a drug repurposing screen, Front Pharmacol
Souza, Carrasco, Rojas-Cortes, Effectiveness of nirmatrelvirritonavir for the treatment of patients with mild to moderate COVID-19 and at high risk of hospitalization: systematic review and meta-analyses of observational studies, PLoS One
Varona, Landete, Lopez-Martin, Preclinical and randomized phase I studies of plitidepsin in adults hospitalized with COVID-19, Life Sci Alliance
Vo, La, Wu, Factors associated with severe COVID-19 among vaccinated adults treated in US Veterans Affairs Hospitals, JAMA Netw Open
White, Rosales, Yildiz, Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A, Science
DOI record:
{
"DOI": "10.1093/cid/ciae227",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciae227",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Plitidepsin has shown potent preclinical activity against severe acute respiratory syndrome coronavirus 2 and was generally well tolerated in a phase I trial of hospitalized patients with coronavirus disease 2019 (COVID-19). NEPTUNO, a phase III, multicenter, randomized, controlled trial, was designed to evaluate the efficacy and safety of plitidepsin in the management of moderate COVID-19 in hospitalized adult patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Included patients had documented severe acute respiratory syndrome coronavirus 2 infection, required oxygen therapy, and had adequate organ function. The planned sample size was 609 patients. Patients were randomized 1:1:1 to at least 3 days of dexamethasone plus either plitidepsin (1.5 mg/day or 2.5 mg/day, for 3 days) or standard of care (control). The primary endpoint was the time to sustained withdrawal of supplemental oxygen. Secondary endpoints included time to sustained hospital discharge, clinical status, duration of oxygen support, percentage of patients requiring admission to the intensive care unit, and safety.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>After randomizing 205 patients, NEPTUNO was discontinued due to a notable drop in COVID-19–related hospitalizations. Available data suggest a 2-day improvement in the median time to sustained oxygen therapy discontinuation (5 vs 7 days) favoring both plitidepsin arms (hazard ratio, 1.37; 95% confidence interval, .96–1.96; P = .08 for plitidepsin 1.5 mg vs control; hazard ratio, 1.06; 95% confidence interval, .73–1.53; P = .78 for plitidepsin 2.5 mg vs control). Plitidepsin was generally well tolerated.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Despite the trial limitations, these results suggest that plitidepsin may have a positive benefit-risk ratio in the management of patients requiring oxygen therapy. Further studies with plitidepsin, including those in immunosuppressed patients, are warranted.</jats:p>\n <jats:p>Results from this phase III trial suggest that plitidepsin, a first-in-class antiviral, may have a positive benefit-risk ratio in the management of hospitalized patients requiring oxygen therapy for moderate COVID-19.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Pneumology Department, Hospital Universitario La Princesa , Madrid ,",
"place": [
"Spain"
]
},
{
"name": "Research Laboratory, Instituto de Investigación La Princesa (IIS Princesa) , Madrid ,",
"place": [
"Spain"
]
},
{
"name": "Department of Pneumology, Hospital Enfermera Isabel Zendal , Madrid SARS CoV2 Unit, Madrid ,",
"place": [
"Spain"
]
},
{
"name": "Department of Pneumology, Universidad Autónoma de Madrid, Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Landete",
"given": "Pedro",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2274-6439",
"affiliation": [
{
"name": "Department of Infectious Diseases, Judetean de Urgenta \"Sf. Ioan cel Nou\" , Suceava ,",
"place": [
"Romania"
]
},
{
"name": "Deparment of Internal Medicine, University of Suceava , Suceava ,",
"place": [
"Romania"
]
}
],
"authenticated-orcid": false,
"family": "Caliman-Sturdza",
"given": "Olga-Adriana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7530-3207",
"affiliation": [
{
"name": "Virology Unit, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"authenticated-orcid": false,
"family": "Lopez-Martin",
"given": "Jose A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3328-4789",
"affiliation": [
{
"name": "Department of Internal Mecicine, Institutul National De Boli Infectioase \"Prof. Dr. Matei Bals\" , Bucharest ,",
"place": [
"Romania"
]
},
{
"name": "Department of Internal Mecicine, University of Medicine and Pharmacy \"Carol Davila\" , Bucharest ,",
"place": [
"Romania"
]
}
],
"authenticated-orcid": false,
"family": "Preotescu",
"given": "Liliana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Spitalul Clinic De Boli Infecţioase \"Sf. Paraschev\" , Iasi ,",
"place": [
"Romania"
]
},
{
"name": "Department of Internal Medicine, \"Grigore T. Popa\" University , Iasi ,",
"place": [
"Romania"
]
}
],
"family": "Luca",
"given": "Mihaela-Catalina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, National and Kapodistrian University of Athens , Athens ,",
"place": [
"Greece"
]
},
{
"name": "Pulmonary and Critical Care, Evagelismos General Hospital , Athens ,",
"place": [
"Greece"
]
}
],
"family": "Kotanidou",
"given": "Anastasia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine, Hospital Universitario HM Sanchinarro, HM Hospitales Group , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Villares",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectiology Department, Clinica de la Costa , Barranquilla ,",
"place": [
"Colombia"
]
}
],
"family": "Iglesias",
"given": "Shirley-Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine, Hospital Universitario Quironsalud Madrid , Madrid ,",
"place": [
"Spain"
]
},
{
"name": "Medical Research Center, Universidad Europea , Madrid, Spain"
}
],
"family": "Guisado-Vasco",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Virology Unit, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Saiz-Lou",
"given": "Elena-Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department, Hospital Universitario ‘Marqués de Valdecilla’ , Santander ,",
"place": [
"Spain"
]
},
{
"name": "Department of Internal Medicine, Valdecilla Research Institute (IDIVAL) , Santander ,",
"place": [
"Spain"
]
},
{
"name": "Department of Medicine and Psychiatry, Universidad de Cantabria , Research Center, Santander ,",
"place": [
"Spain"
]
}
],
"family": "del Carmen Farinas-Alvarez",
"given": "Maria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3854-4874",
"affiliation": [
{
"name": "Unit of Infectious Diseases, Alicante General University Hospital , Alicante ,",
"place": [
"Spain"
]
},
{
"name": "Department of Infectious Disease, Alicante Institute of Health and Biomedical Research (ISABIAL) , Alicante ,",
"place": [
"Spain"
]
}
],
"authenticated-orcid": false,
"family": "de Lucas",
"given": "Esperanza Merino",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectology Department, Hospital Universitario \"Dr. José Eleuterio González\" , Monterrey ,",
"place": [
"Mexico"
]
},
{
"name": "Department of Infectious Diseases, Universidad Autónoma de Nuevo León , Monterrey ,",
"place": [
"Mexico"
]
}
],
"family": "Perez-Alba",
"given": "Eduardo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5001-672X",
"affiliation": [
{
"name": "Department of Research, Institute of Biomedicine of Seville (IBiS) , Seville ,",
"place": [
"Spain"
]
},
{
"name": "Department of Infectious Diseases, Virgen del Rocío’ University Hospital , Seville ,",
"place": [
"Spain"
]
}
],
"authenticated-orcid": false,
"family": "Cisneros",
"given": "Jose-Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Hospital Universitario \"San Carlos\" , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Estrada",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Hospital Universitario \"Virgen de las Nieves\" , Granada ,",
"place": [
"Spain"
]
}
],
"family": "Hidalgo-Tenorio",
"given": "Carmen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0778-0006",
"affiliation": [
{
"name": "3rd Department of Internal Medicine and Laboratory, National Sotiria General Hospital, Athens ,",
"place": [
"Greece"
]
}
],
"authenticated-orcid": false,
"family": "Poulakou",
"given": "Garyfallia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2166-7405",
"affiliation": [
{
"name": "Internal Medicine, Guadalajara University Hospital , Guadalajara ,",
"place": [
"Spain"
]
}
],
"authenticated-orcid": false,
"family": "Torralba",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department, Hospital Universitario Ramon y Cajal , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Fortun",
"given": "Jesus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Hospital Universitario de Jerez de la Frontera , Cádiz ,",
"place": [
"Spain"
]
}
],
"family": "Garcia-Ocana",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Centre Hospitalier Regional et Universitaire de Tours (CHRU Tours)—Hopital Bretonneaut , Tours ,",
"place": [
"France"
]
}
],
"family": "Lemaignen",
"given": "Adrien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Hospital Universitario de Salamanca , Salamanca ,",
"place": [
"Spain"
]
}
],
"family": "Marcos-Martin",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ILD Unit-Respiratory Department, University Hospital of Bellvitge , Barcelona ,",
"place": [
"Spain"
]
},
{
"name": "Bellvitge Institute for Biomedical Reseach, IDIBELL , Barcelona ,",
"place": [
"Spain"
]
},
{
"name": "Department of Research Center, CIBERES , Barcelona ,",
"place": [
"Spain"
]
}
],
"family": "Molina",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department, Hospital Universitari Germans Trial I Pujol , Badalona ,",
"place": [
"Spain"
]
},
{
"name": "Department of Infectious Diseases, IrsiCaixa AIDS Research Institute , Badalona ,",
"place": [
"Spain"
]
}
],
"family": "Paredes",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, Complexo Hospitalario Universitario de Vigo . Vigo ,",
"place": [
"Spain"
]
}
],
"family": "Perez-Rodriguez",
"given": "Maria Teresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cardiology and Internal Medicine, Internal Medicine Clinic, University Hospital UMHAT “Sveta Anna”, Sofia ,",
"place": [
"Bulgaria"
]
}
],
"family": "Raev",
"given": "Dimitar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Hospital Infanta Leonor , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Ryan",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Virology Unit, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Meira",
"given": "Fernanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Gomez",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Data Management, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Torres",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Virology Unit, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"family": "Lopez-Mendoza",
"given": "Diego",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0004-1095-1990",
"affiliation": [
{
"name": "Virology Unit, PharmaMar , Madrid ,",
"place": [
"Spain"
]
}
],
"authenticated-orcid": false,
"family": "Jimeno",
"given": "Jose",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4764-9440",
"affiliation": [
{
"name": "Department of Internal Medicine, Hospital Universitario HM Monteprincipe, HM Hospitales , Madrid ,",
"place": [
"Spain"
]
},
{
"name": "Facultad HM de Ciencias de la Salud, Universidad Camilo Jose Cela, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Varona",
"given": "Jose-Felipe",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
8,
26
]
],
"date-time": "2024-08-26T00:54:28Z",
"timestamp": 1724633668000
},
"deposited": {
"date-parts": [
[
2024,
10,
15
]
],
"date-time": "2024-10-15T19:07:49Z",
"timestamp": 1729019269000
},
"funder": [
{
"name": "Pharma Mar S.A"
}
],
"indexed": {
"date-parts": [
[
2024,
10,
16
]
],
"date-time": "2024-10-16T04:25:33Z",
"timestamp": 1729052733611
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
8,
26
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2024,
8,
26
]
]
},
"published-print": {
"date-parts": [
[
2024,
10,
15
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
26
]
],
"date-time": "2024-08-26T00:00:00Z",
"timestamp": 1724630400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae227/59140735/ciae227.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/79/4/910/59764870/ciae227.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/79/4/910/59764870/ciae227.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "910-919",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
8,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
26
]
]
},
"published-other": {
"date-parts": [
[
2024,
10,
15
]
]
},
"published-print": {
"date-parts": [
[
2024,
10,
15
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1038/srep35100",
"article-title": "Translation elongation factor eEF1A2 is a novel anticancer target for the marine natural product plitidepsin",
"author": "Losada",
"doi-asserted-by": "crossref",
"first-page": "35100",
"journal-title": "Sci Rep",
"key": "2024101519060021700_ciae227-B1",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.26508/lsa.202101200",
"article-title": "Preclinical and randomized phase I studies of plitidepsin in adults hospitalized with COVID-19",
"author": "Varona",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci Alliance",
"key": "2024101519060021700_ciae227-B2",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1126/science.abf4058",
"article-title": "Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A",
"author": "White",
"doi-asserted-by": "crossref",
"first-page": "926",
"journal-title": "Science",
"key": "2024101519060021700_ciae227-B3",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.646676",
"article-title": "Identification of plitidepsin as potent inhibitor of SARS-CoV-2-induced cytopathic effect after a drug repurposing screen",
"author": "Rodon",
"doi-asserted-by": "crossref",
"first-page": "646676",
"journal-title": "Front Pharmacol",
"key": "2024101519060021700_ciae227-B4",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2023.08.026",
"article-title": "SARS-CoV-2 variants evolve convergent strategies to remodel the host response",
"author": "Bouhaddou",
"doi-asserted-by": "crossref",
"first-page": "4597",
"journal-title": "Cell",
"key": "2024101519060021700_ciae227-B5",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "WHO Working Group on the Clinical Characterisation and Management of Covid-infection",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "2024101519060021700_ciae227-B6",
"volume": "20",
"year": "2020"
},
{
"article-title": "Functional evaluation: the Barthel Index",
"author": "Mahoney",
"first-page": "61",
"journal-title": "Md State Med J",
"key": "2024101519060021700_ciae227-B7",
"volume": "14",
"year": "1965"
},
{
"DOI": "10.1016/0895-4356(94)90129-5",
"article-title": "Validation of a combined comorbidity index",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "J Clin Epidemiol",
"key": "2024101519060021700_ciae227-B8",
"volume": "47",
"year": "1994"
},
{
"DOI": "10.1016/j.ebiom.2022.104008",
"article-title": "The displacement of the SARS-CoV-2 variant Delta with Omicron: an investigation of hospital admissions and upper respiratory viral loads",
"author": "Fall",
"doi-asserted-by": "crossref",
"first-page": "104008",
"journal-title": "EBioMedicine",
"key": "2024101519060021700_ciae227-B9",
"volume": "79",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0284006",
"article-title": "Effectiveness of nirmatrelvir-ritonavir for the treatment of patients with mild to moderate COVID-19 and at high risk of hospitalization: systematic review and meta-analyses of observational studies",
"author": "Souza",
"doi-asserted-by": "crossref",
"first-page": "e0284006",
"journal-title": "PLoS One",
"key": "2024101519060021700_ciae227-B10",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2022.40037",
"article-title": "Factors associated with severe COVID-19 among vaccinated adults treated in US Veterans Affairs Hospitals",
"author": "Vo",
"doi-asserted-by": "crossref",
"first-page": "e2240037",
"journal-title": "JAMA Netw Open",
"key": "2024101519060021700_ciae227-B11",
"volume": "5",
"year": "2022"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2024101519060021700_ciae227-B12"
},
{
"DOI": "10.1016/j.ijid.2023.07.011",
"article-title": "Outcomes and clinical characteristics of the compassionate use of plitidepsin for immunocompromised adult patients with COVID-19",
"author": "Aguareles",
"doi-asserted-by": "crossref",
"first-page": "12",
"journal-title": "Int J Infect Dis",
"key": "2024101519060021700_ciae227-B13",
"volume": "135",
"year": "2023"
}
],
"reference-count": 13,
"references-count": 13,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/article/79/4/910/7740910"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Phase III Randomized Controlled Trial of Plitidepsin, a Marine-Derived Compound, in Hospitalized Adults With Moderate COVID-19",
"type": "journal-article",
"volume": "79"
}