Efficacy of three antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the saliva of hospitalized patients: a randomized controlled pilot study
et al., Scientific Reports, doi:10.1038/s41598-023-39308-x, NCT04723446, Aug 2023
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 40 late stage (mean 8 days from onset) patients, showing no significant difference in short-term viral load measured by PCR with cetylpyridinium chloride mouthwash.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
Study covers cetylpyridinium chloride and hydrogen peroxide.
Perussolo et al., 4 Aug 2023, Single Blind Randomized Controlled Trial, United Kingdom, peer-reviewed, mean age 42.6, 7 authors, study period April 2021 - October 2021, average treatment delay 8.7 days, trial NCT04723446 (history).
Efficacy of three antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the saliva of hospitalized patients: a randomized controlled pilot study
Scientific Reports, doi:10.1038/s41598-023-39308-x
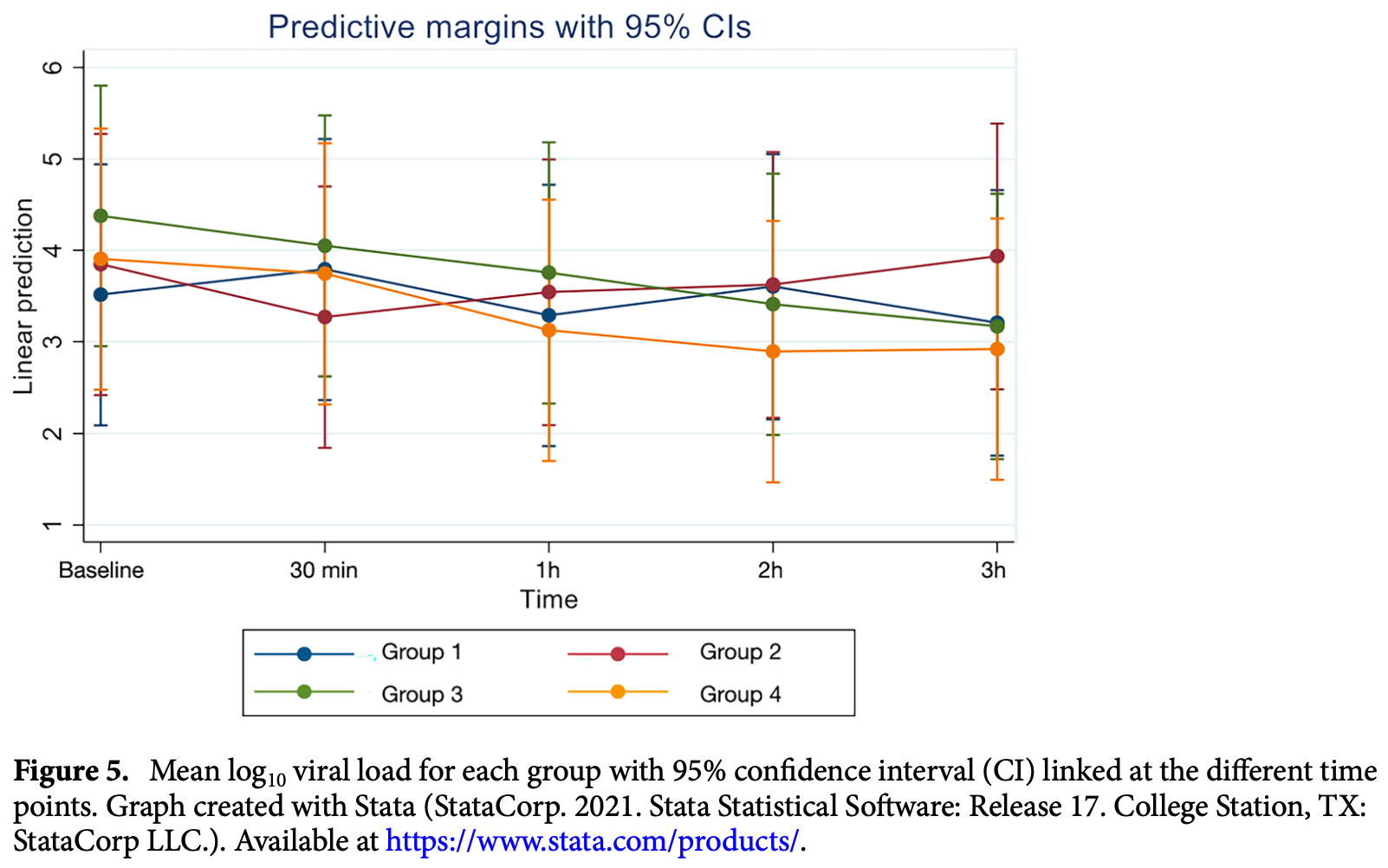
This study aimed to evaluate the efficacy of 3 mouthwashes in reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in the saliva of coronavirus disease 2019 (COVID-19) patients at 30 min, 1, 2 and 3 h after rinsing. This pilot study included 40 admitted COVID-19 positive patients (10 in each group). Saliva samples were collected before rinsing and at 30 min, 1, 2 and 3 h after rinsing with: Group 1-0.2% Chlorhexidine digluconate (CHX); Group 2-1.5% Hydrogen peroxide (H 2 O 2 ); Group 3-Cetylpyridinium chloride (CPC) or Group 4 (control group)-No rinsing. Viral load analysis of saliva samples was assessed by Reverse Transcription quantitative PCR. Mean log 10 viral load at different time points was compared to that at baseline in all groups using a random effects linear regression analysis while for comparison between groups linear regression analysis was used. The results showed that all groups had a significantly reduced mean log 10 viral load both at 2 (p = 0.036) and 3 (p = 0.041) hours compared to baseline. However, there was no difference in mean log 10 viral load between any of the investigated mouthwashes and the control group (non-rinsing) at the evaluated time points. Although a reduction in the SARS-CoV-2 viral load in the saliva of COVID-19 patients was observed after rinsing with mouthwashes containing 0.2% CHX, 1.5% H 2 O 2 , or CPC, the reduction detected was similar to that achieved by the control group at the investigated time points. The findings of this study may suggest that the mechanical action of rinsing/spitting results in reduction of SARS-CoV-2 salivary load. Coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also commonly known as coronavirus, was declared a pandemic in 2020 by the World Health Organization (WHO) presenting with more than 500 million confirmed cases and 6 million deaths worldwide 1 . COVID-19 is characterized by an unpredictable disease course, ranging from asymptomatic to severe, lifethreatening infections 2 . SARS-CoV-2, part of a group of 'enveloped viruses' characterized by an outer lipid membrane 3 , has been detected in various clinical specimens such as saliva, throat, nasopharyngeal (NPS), and oropharyngeal (OPS) swabs, and bronchoalveolar-lavage fluid 4 . Angiotensin-converting enzyme II (ACE2), a cell receptor for SARS-CoV which plays an important role in the entry of the virus into the cell, is highly expressed in the oral cavity and oral epithelial cells 5 . A study by To et al. 6 demonstrated SARS-CoV-2 being detected in 91.7% of the saliva samples obtained from COVID-19 positive patients. In addition, a recent study has further shown that detection rate of SARS-CoV-2 virus in saliva samples can be even higher than that on NPS (93.1%
Author contributions N.D. was responsible for the concept of the study. J.P., N.G., M.T., and N.D. designed the trial and study protocol. J.P. was responsible for the site work including the recruitment, study visits and data collection. S.T. facilitated recruitment. S.T. and M.C.M. provided relevant medical advice and information. M.T. performed the viral load analysis. J.P., N.G., M.T., S.T., A.P., and N.D. contributed to the data interpretation. A.P. did the main statistical analysis. J.P., N.G., A.P., M.T., S.T., and N.D. prepared the manuscript. All authors reviewed and accepted the paper before submission.
Competing interests N.D. had an advisory role at GlaxoSmithKline (GSK) and was awarded a GSK Consumer Healthcare (GSKCH) research grant, which provided funding for this study. ND has also lectured for Oral-B. S.T. is a GSK Global Health Consultant. J.P., N.G., A.P., M.T. and M.C.M. do not report any competing interest.
Additional information
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1038/ s41598-023-39308-x. Correspondence and requests for materials should be addressed to N.D. Reprints and permissions information is available at www.nature.com/reprints. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Andersson, SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus, Wellcome Open Res, doi:10.12688/wellcomeopenres.16002.2
Bernstein, In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse, J. Dent. Res, doi:10.1177/00220345900690030901
Biber, The role of mouthwash sampling in SARS-CoV-2 diagnosis, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/s10096-021-04320-4
Blanco-Melo, Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Caruso, Del, Lazzarino, Hydrogen peroxide and viral infections: A literature review with research hypothesis definition in relation to the current covid-19 pandemic, Med. Hypotheses, doi:10.1016/j.mehy.2020.109910
Challenger, Modelling upper respiratory viral load dynamics of SARS-CoV-2, BMC Med, doi:10.1186/s12916-021-02220-0
Chaudhary, Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial, J. Am. Dent. Assoc, doi:10.1016/j.adaj.2021.05.021
Crocker-Buque, The Barts Health NHS Trust COVID-19 cohort: characteristics, outcomes and risk scoring of patients in East London, Int. J. Tuberc. Lung Dis, doi:10.5588/ijtld.20.0926
Eduardo, Effectiveness of toothpastes on SARS-CoV-2 viral load in saliva, Int. Dent. J, doi:10.1016/j.identj.2022.03.006
Eduardo, Salivary SARS-CoV-2 load reduction with mouthwash use : A randomized pilot clinical trial, Heliyon, doi:10.1016/j.heliyon.2021.e07346
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA), Infect. Dis. Ther, doi:10.1007/s40121-015-0091-9
Elzein, In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-COV-2. A randomized-controlled clinical trial, J. Evid. Based Dent. Pract, doi:10.1016/j.jebdp.2021.101584
Ferrer, Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Sci. Rep, doi:10.1038/s41598-021-03461-y
Gandhi, Thimmappa, Upadhya, Carnelio, Could mouth rinses be an adjuvant in the treatment of SARS-CoV-2 patients? An appraisal with a systematic review, Int. J. Dent. Hyg, doi:10.1111/idh.12555
Garcia-Sanchez, Efficacy of pre-procedural mouthwashes against SARS-CoV-2: A systematic review of randomized controlled trials, J. Clin. Med, doi:10.3390/jcm11061692
Gottsauner, A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clin. Oral. Investig, doi:10.1007/s00784-020-03549-1
Green, In vitro assessment of the virucidal activity of four mouthwashes containing Cetylpyridinium Chloride, ethanol, zinc and a mix of enzyme and proteins against a human coronavirus, doi:10.1101/2020.10.28.359257
Guest, Suitability and Sufficiency of telehealth clinician-observed participant-collected samples for SARS-CoV2 testing: the iCollect Cohort Pilot Study, J. Med. Internet Res, doi:10.2196/19731
Health, Understanding cycle threshold (Ct) in SARS-CoV-2 RT-PCR A guide for health protection teams
Hernández-Vásquez, Barrenechea-Pulache, Comandé, Azañedo, Mouthrinses and SARS-CoV-2 viral load in saliva: A living systematic review, Evid. Based Dent, doi:10.1038/s41432-022-0253-z
Kampf, Todt, Pfaender, Steinmann, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, J. Hosp. Infect, doi:10.1016/j.jhin.2020.01.022
Kronbichler, Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.06.052
Meister, Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J. Infect. Dis, doi:10.1093/infdis/jiaa471
Miranda, Guterres, De Azeredo Lima, Filho, Gadelha, Misinterpretation of viral load in COVID-19 clinical outcomes, Virus Res, doi:10.1016/j.virusres.2021.198340
O'donnell, Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, doi:10.1093/function/zqaa002
Ortega, Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review, J. Hosp. Infect, doi:10.1016/j.jhin.2020.10.003
Pan, Transmission routes of SARS-CoV-2 and protective measures in dental clinics during the COVID-19 pandemic, Am. J. Dent
Popkin, Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathog. Immun, doi:10.20411/pai.v2i2.200
Rao, Comparing nasopharyngeal swab and early morning saliva for the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa1156
Seneviratne, Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore, Infection, doi:10.1007/s15010-020-01563-9
Silva, Azevedo, Sampaio-Maia, Sousa-Pinto, The effect of mouthrinses on severe acute respiratory syndrome coronavirus 2 viral load: A systematic review, J. Am. Dent. Assoc, doi:10.1016/j.adaj.2021.12.007
Tartaglia, Tadakamadla, Connelly, Sforza, Martín, Adverse events associated with home use of mouthrinses: A systematic review, Ther. Adv. Drug Saf, doi:10.1177/2042098619854881
To, Consistent detection of 2019 novel coronavirus in saliva, Clin. Infect. Dis, doi:10.1093/cid/ciaa149
Urbaniak, Plous, Randomizer, None
Wölfel, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Xu, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int. J. Oral Sci, doi:10.1038/s41368-020-0074-x
Yoon, Clinical significance of a high SARS-CoV-2 viral load in the saliva, J. Korean Med. Sci, doi:10.3346/jkms.2020.35.e195
Ziegler, SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues, Cell, doi:10.1016/j.cell.2020.04.035
DOI record:
{
"DOI": "10.1038/s41598-023-39308-x",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-023-39308-x",
"abstract": "<jats:title>Abstract</jats:title><jats:p>This study aimed to evaluate the efficacy of 3 mouthwashes in reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in the saliva of coronavirus disease 2019 (COVID-19) patients at 30 min, 1, 2 and 3 h after rinsing. This pilot study included 40 admitted COVID-19 positive patients (10 in each group). Saliva samples were collected before rinsing and at 30 min, 1, 2 and 3 h after rinsing with: <jats:italic>Group 1</jats:italic>—0.2% Chlorhexidine digluconate (CHX); <jats:italic>Group 2</jats:italic>—1.5% Hydrogen peroxide (H<jats:sub>2</jats:sub>O<jats:sub>2</jats:sub>); <jats:italic>Group 3</jats:italic>—Cetylpyridinium chloride (CPC) or <jats:italic>Group 4</jats:italic> (control group)—No rinsing. Viral load analysis of saliva samples was assessed by Reverse Transcription quantitative PCR. Mean log<jats:sub>10</jats:sub> viral load at different time points was compared to that at baseline in all groups using a random effects linear regression analysis while for comparison between groups linear regression analysis was used. The results showed that all groups had a significantly reduced mean log<jats:sub>10</jats:sub> viral load both at 2 (<jats:italic>p</jats:italic> = 0.036) and 3 (<jats:italic>p</jats:italic> = 0.041) hours compared to baseline. However, there was no difference in mean log<jats:sub>10</jats:sub> viral load between any of the investigated mouthwashes and the control group (non-rinsing) at the evaluated time points. Although a reduction in the SARS-CoV-2 viral load in the saliva of COVID-19 patients was observed after rinsing with mouthwashes containing 0.2% CHX, 1.5% H<jats:sub>2</jats:sub>O<jats:sub>2</jats:sub>, or CPC, the reduction detected was similar to that achieved by the control group at the investigated time points. The findings of this study may suggest that the mechanical action of rinsing/spitting results in reduction of SARS-CoV-2 salivary load.</jats:p>",
"alternative-id": [
"39308"
],
"article-number": "12647",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "29 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "4 August 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "N.D. had an advisory role at GlaxoSmithKline (GSK) and was awarded a GSK Consumer Healthcare (GSKCH) research grant, which provided funding for this study. ND has also lectured for Oral-B. S.T. is a GSK Global Health Consultant. J.P., N.G., A.P., M.T. and M.C.M. do not report any competing interest."
}
],
"author": [
{
"affiliation": [],
"family": "Perussolo",
"given": "Jeniffer",
"sequence": "first"
},
{
"affiliation": [],
"family": "Teh",
"given": "Muy-Teck",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gkranias",
"given": "Nikolaos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tiberi",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petrie",
"given": "Aviva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cutino-Moguel",
"given": "Maria-Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donos",
"given": "Nikolaos",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
8,
4
]
],
"date-time": "2023-08-04T10:02:18Z",
"timestamp": 1691143338000
},
"deposited": {
"date-parts": [
[
2023,
8,
4
]
],
"date-time": "2023-08-04T10:10:37Z",
"timestamp": 1691143837000
},
"indexed": {
"date-parts": [
[
2023,
8,
5
]
],
"date-time": "2023-08-05T04:27:39Z",
"timestamp": 1691209659307
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
8,
4
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
4
]
],
"date-time": "2023-08-04T00:00:00Z",
"timestamp": 1691107200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
4
]
],
"date-time": "2023-08-04T00:00:00Z",
"timestamp": 1691107200000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-023-39308-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-39308-x",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-39308-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
8,
4
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
4
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "39308_CR1",
"unstructured": "World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. (Accessed 2022); https://covid19.who.int/"
},
{
"DOI": "10.1016/j.ijid.2020.06.052",
"author": "A Kronbichler",
"doi-asserted-by": "publisher",
"first-page": "180",
"journal-title": "Int. J. Infect. Dis.",
"key": "39308_CR2",
"unstructured": "Kronbichler, A. et al. Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 98, 180–186. https://doi.org/10.1016/j.ijid.2020.06.052 (2020).",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1093/function/zqaa002",
"author": "VB O’Donnell",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Function",
"key": "39308_CR3",
"unstructured": "O’Donnell, V. B. et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function 1, 1–12. https://doi.org/10.1093/function/zqaa002 (2020).",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.12688/wellcomeopenres.16002.2",
"author": "MI Andersson",
"doi-asserted-by": "publisher",
"journal-title": "Wellcome Open Res.",
"key": "39308_CR4",
"unstructured": "Andersson, M. I. et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.16002.2 (2020).",
"year": "2020"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"author": "H Xu",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Int. J. Oral Sci.",
"key": "39308_CR5",
"unstructured": "Xu, H. et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 12, 8. https://doi.org/10.1038/s41368-020-0074-x (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa149",
"author": "KK To",
"doi-asserted-by": "publisher",
"first-page": "4",
"journal-title": "Clin. Infect. Dis.",
"key": "39308_CR6",
"unstructured": "To, K. K. et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020, 4–6. https://doi.org/10.1093/cid/ciaa149 (2020).",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1156",
"author": "M Rao",
"doi-asserted-by": "publisher",
"first-page": "352",
"journal-title": "Clin. Infect. Dis.",
"key": "39308_CR7",
"unstructured": "Rao, M. et al. Comparing nasopharyngeal swab and early morning saliva for the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2, 352–356. https://doi.org/10.1093/cid/ciaa1156 (2021).",
"volume": "2",
"year": "2021"
},
{
"author": "Y Pan",
"first-page": "129",
"issue": "3",
"journal-title": "Am. J. Dent.",
"key": "39308_CR8",
"unstructured": "Pan, Y. et al. Transmission routes of SARS-CoV-2 and protective measures in dental clinics during the COVID-19 pandemic. Am. J. Dent. 33(3), 129–134 (2020).",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1016/j.jhin.2020.01.022",
"author": "G Kampf",
"doi-asserted-by": "publisher",
"first-page": "246",
"issue": "3",
"journal-title": "J. Hosp. Infect.",
"key": "39308_CR9",
"unstructured": "Kampf, G., Todt, D., Pfaender, S. & Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 104(3), 246–251. https://doi.org/10.1016/j.jhin.2020.01.022 (2020).",
"volume": "104",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109910",
"author": "AA Caruso",
"doi-asserted-by": "publisher",
"first-page": "109910",
"journal-title": "Med. Hypotheses",
"key": "39308_CR10",
"unstructured": "Caruso, A. A., Del, P. A. & Lazzarino, A. I. Hydrogen peroxide and viral infections: A literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med. Hypotheses 144, 109910. https://doi.org/10.1016/j.mehy.2020.109910 (2020).",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1016/j.identj.2022.03.006",
"author": "FP Eduardo",
"doi-asserted-by": "publisher",
"first-page": "825",
"issue": "6",
"journal-title": "Int. Dent. J.",
"key": "39308_CR11",
"unstructured": "Eduardo, F. P. et al. Effectiveness of toothpastes on SARS-CoV-2 viral load in saliva. Int. Dent. J. 72(6), 825–831. https://doi.org/10.1016/j.identj.2022.03.006 (2022).",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/j.heliyon.2021.e07346",
"author": "FP Eduardo",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "June",
"journal-title": "Heliyon",
"key": "39308_CR12",
"unstructured": "Eduardo, F. P. et al. Salivary SARS-CoV-2 load reduction with mouthwash use : A randomized pilot clinical trial. Heliyon 7(June), 1–7. https://doi.org/10.1016/j.heliyon.2021.e07346 (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1177/2042098619854881",
"author": "GM Tartaglia",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Ther. Adv. Drug Saf.",
"key": "39308_CR13",
"unstructured": "Tartaglia, G. M., Tadakamadla, S. K., Connelly, S. T., Sforza, C. & Martín, C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 10, 1–16. https://doi.org/10.1177/2042098619854881 (2019).",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "491",
"issue": "4",
"journal-title": "Infect. Dis. Ther.",
"key": "39308_CR14",
"unstructured": "Eggers, M., Eickmann, M. & Zorn, J. Rapid and effective virucidal activity of povidone- iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA). Infect. Dis. Ther. 4(4), 491–501. https://doi.org/10.1007/s40121-015-0091-9 (2015).",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1177/00220345900690030901",
"author": "D Bernstein",
"doi-asserted-by": "publisher",
"first-page": "874",
"issue": "3",
"journal-title": "J. Dent. Res.",
"key": "39308_CR15",
"unstructured": "Bernstein, D. et al. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J. Dent. Res. 69(3), 874–876. https://doi.org/10.1177/00220345900690030901 (1990).",
"volume": "69",
"year": "1990"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"author": "JG Yoon",
"doi-asserted-by": "publisher",
"first-page": "e195",
"issue": "20",
"journal-title": "J. Korean Med. Sci.",
"key": "39308_CR16",
"unstructured": "Yoon, J. G. et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J. Korean Med. Sci. 35(20), e195. https://doi.org/10.3346/jkms.2020.35.e195 (2020).",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.20411/pai.v2i2.200",
"author": "DL Popkin",
"doi-asserted-by": "publisher",
"first-page": "252",
"issue": "2",
"journal-title": "Pathog. Immun.",
"key": "39308_CR17",
"unstructured": "Popkin, D. L. et al. Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo. Pathog. Immun. 2(2), 252–269. https://doi.org/10.20411/pai.v2i2.200 (2017).",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1101/2020.10.28.359257",
"author": "A Green",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "39308_CR18",
"unstructured": "Green, A. et al. In vitro assessment of the virucidal activity of four mouthwashes containing Cetylpyridinium Chloride, ethanol, zinc and a mix of enzyme and proteins against a human coronavirus. bioRxiv https://doi.org/10.1101/2020.10.28.359257 (2020).",
"year": "2020"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"author": "MJ Gottsauner",
"doi-asserted-by": "publisher",
"first-page": "3707",
"issue": "10",
"journal-title": "Clin. Oral. Investig.",
"key": "39308_CR19",
"unstructured": "Gottsauner, M. J. et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral. Investig. 24(10), 3707–3713. https://doi.org/10.1007/s00784-020-03549-1 (2020).",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"author": "CJ Seneviratne",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "2",
"journal-title": "Infection",
"key": "39308_CR20",
"unstructured": "Seneviratne, C. J. et al. Efficacy of commercial mouth—rinses on SARS—CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 49(2), 305–311. https://doi.org/10.1007/s15010-020-01563-9 (2021).",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1111/idh.12555",
"author": "G Gandhi",
"doi-asserted-by": "publisher",
"first-page": "136",
"issue": "1",
"journal-title": "Int. J. Dent. Hyg.",
"key": "39308_CR21",
"unstructured": "Gandhi, G., Thimmappa, L., Upadhya, N. & Carnelio, S. Could mouth rinses be an adjuvant in the treatment of SARS-CoV-2 patients? An appraisal with a systematic review. Int. J. Dent. Hyg. 20(1), 136–144. https://doi.org/10.1111/idh.12555 (2022).",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/j.adaj.2021.12.007",
"author": "A Silva",
"doi-asserted-by": "publisher",
"first-page": "635",
"issue": "7",
"journal-title": "J. Am. Dent. Assoc.",
"key": "39308_CR22",
"unstructured": "Silva, A., Azevedo, M., Sampaio-Maia, B. & Sousa-Pinto, B. The effect of mouthrinses on severe acute respiratory syndrome coronavirus 2 viral load: A systematic review. J. Am. Dent. Assoc. 153(7), 635-648.e16. https://doi.org/10.1016/j.adaj.2021.12.007 (2022).",
"volume": "153",
"year": "2022"
},
{
"DOI": "10.3390/jcm11061692",
"author": "A Garcia-Sanchez",
"doi-asserted-by": "publisher",
"journal-title": "J. Clin. Med.",
"key": "39308_CR23",
"unstructured": "Garcia-Sanchez, A. et al. Efficacy of pre-procedural mouthwashes against SARS-CoV-2: A systematic review of randomized controlled trials. J. Clin. Med. https://doi.org/10.3390/jcm11061692 (2022).",
"year": "2022"
},
{
"DOI": "10.1016/j.jhin.2020.10.003",
"author": "KL Ortega",
"doi-asserted-by": "publisher",
"first-page": "657",
"issue": "4",
"journal-title": "J. Hosp. Infect.",
"key": "39308_CR24",
"unstructured": "Ortega, K. L. et al. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. J. Hosp. Infect. 106(4), 657–662. https://doi.org/10.1016/j.jhin.2020.10.003 (2020).",
"volume": "106",
"year": "2020"
},
{
"DOI": "10.1038/s41432-022-0253-z",
"author": "A Hernández-Vásquez",
"doi-asserted-by": "publisher",
"journal-title": "Evid. Based Dent.",
"key": "39308_CR25",
"unstructured": "Hernández-Vásquez, A., Barrenechea-Pulache, A., Comandé, D. & Azañedo, D. Mouthrinses and SARS-CoV-2 viral load in saliva: A living systematic review. Evid. Based Dent. https://doi.org/10.1038/s41432-022-0253-z (2022).",
"year": "2022"
},
{
"DOI": "10.5588/ijtld.20.0926",
"author": "T Crocker-Buque",
"doi-asserted-by": "publisher",
"first-page": "358",
"issue": "5",
"journal-title": "Int. J. Tuberc. Lung Dis.",
"key": "39308_CR26",
"unstructured": "Crocker-Buque, T. et al. The Barts Health NHS Trust COVID-19 cohort: characteristics, outcomes and risk scoring of patients in East London. Int. J. Tuberc. Lung Dis. 25(5), 358–366. https://doi.org/10.5588/ijtld.20.0926 (2021).",
"volume": "25",
"year": "2021"
},
{
"key": "39308_CR27",
"unstructured": "Urbaniak, G., & Plous, S. Research Randomizer (Version 4.0) [Computer software] (2013)."
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet",
"key": "39308_CR28",
"unstructured": "RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 397(10285), 1637–1645. https://doi.org/10.1016/S0140-6736(21)00676-0 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.adaj.2021.05.021",
"author": "P Chaudhary",
"doi-asserted-by": "publisher",
"first-page": "903",
"issue": "11",
"journal-title": "J. Am. Dent. Assoc.",
"key": "39308_CR29",
"unstructured": "Chaudhary, P. et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial. J. Am. Dent. Assoc. 152(11), 903–908. https://doi.org/10.1016/j.adaj.2021.05.021 (2021).",
"volume": "152",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"author": "MD Ferrer",
"doi-asserted-by": "publisher",
"first-page": "24392",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "39308_CR30",
"unstructured": "Ferrer, M. D. et al. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Sci. Rep. 11(1), 24392. https://doi.org/10.1038/s41598-021-03461-y (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiaa471",
"author": "TL Meister",
"doi-asserted-by": "publisher",
"journal-title": "J. Infect. Dis.",
"key": "39308_CR31",
"unstructured": "Meister, T. L. et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa471 (2020).",
"year": "2020"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"author": "R Elzein",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "3",
"journal-title": "J. Evid. Based Dent. Pract.",
"key": "39308_CR32",
"unstructured": "Elzein, R. et al. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-COV-2. A randomized-controlled clinical trial. J. Evid. Based Dent. Pract. 21(3), 1–10. https://doi.org/10.1016/j.jebdp.2021.101584 (2021).",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2021.198340",
"author": "RL Miranda",
"doi-asserted-by": "publisher",
"first-page": "198340",
"journal-title": "Virus Res.",
"key": "39308_CR33",
"unstructured": "Miranda, R. L., Guterres, A., de Azeredo Lima, C. H., Filho, P. N. & Gadelha, M. R. Misinterpretation of viral load in COVID-19 clinical outcomes. Virus Res. 296, 198340. https://doi.org/10.1016/j.virusres.2021.198340 (2021).",
"volume": "296",
"year": "2021"
},
{
"DOI": "10.2196/19731",
"author": "JL Guest",
"doi-asserted-by": "publisher",
"first-page": "e19731",
"issue": "2",
"journal-title": "J. Med. Internet Res.",
"key": "39308_CR34",
"unstructured": "Guest, J. L. et al. Suitability and Sufficiency of telehealth clinician-observed participant- collected samples for SARS-CoV2 testing: the iCollect Cohort Pilot Study. J. Med. Internet Res. 6(2), e19731. https://doi.org/10.2196/19731 (2020).",
"volume": "6",
"year": "2020"
},
{
"key": "39308_CR35",
"unstructured": "Public Health England. Understanding cycle threshold (Ct) in SARS-CoV-2 RT-PCR A guide for health protection teams. (2022) https://www.gov.uk/government/publications/cycle-threshold-ct-in-sars-cov-2-rt-pcr"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"author": "R Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"issue": "7809",
"journal-title": "Nature",
"key": "39308_CR36",
"unstructured": "Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581(7809), 465–469. https://doi.org/10.1038/s41586-020-2196-x (2020).",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.035",
"author": "CGK Ziegler",
"doi-asserted-by": "publisher",
"first-page": "1016",
"issue": "5",
"journal-title": "Cell",
"key": "39308_CR37",
"unstructured": "Ziegler, C. G. K. et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181(5), 1016-1035.e19. https://doi.org/10.1016/j.cell.2020.04.035 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"author": "D Blanco-Melo",
"doi-asserted-by": "publisher",
"first-page": "1036",
"issue": "5",
"journal-title": "Cell",
"key": "39308_CR38",
"unstructured": "Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181(5), 1036-1045.e9. https://doi.org/10.1016/j.cell.2020.04.026 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1186/s12916-021-02220-0",
"author": "JD Challenger",
"doi-asserted-by": "publisher",
"first-page": "25",
"issue": "1",
"journal-title": "BMC Med.",
"key": "39308_CR39",
"unstructured": "Challenger, J. D. et al. Modelling upper respiratory viral load dynamics of SARS-CoV-2. BMC Med. 20(1), 25. https://doi.org/10.1186/s12916-021-02220-0 (2022).",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1007/s10096-021-04320-4",
"author": "A Biber",
"doi-asserted-by": "publisher",
"first-page": "2199",
"issue": "10",
"journal-title": "Eur. J. Clin. Microbiol. Infect. Dis.",
"key": "39308_CR40",
"unstructured": "Biber, A. et al. The role of mouthwash sampling in SARS-CoV-2 diagnosis. Eur. J. Clin. Microbiol. Infect. Dis. 40(10), 2199–2206. https://doi.org/10.1007/s10096-021-04320-4 (2021).",
"volume": "40",
"year": "2021"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-023-39308-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Efficacy of three antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the saliva of hospitalized patients: a randomized controlled pilot study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}
perussolo
