Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore
et al., Infection, doi:10.1007/s15010-020-01563-9, Dec 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small mouthwash RCT with 4 PVP-I patients and 2 water patients concluding that PVP-I may have a sustained effect on reducing the salivary SARS-CoV-2 level in COVID-19 patients. ISRCTN95933274.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
This study is excluded in the after exclusion results of meta-analysis:
study only provides short-term viral load results.
Study covers chlorhexidine, cetylpyridinium chloride, and povidone-iodine.
|
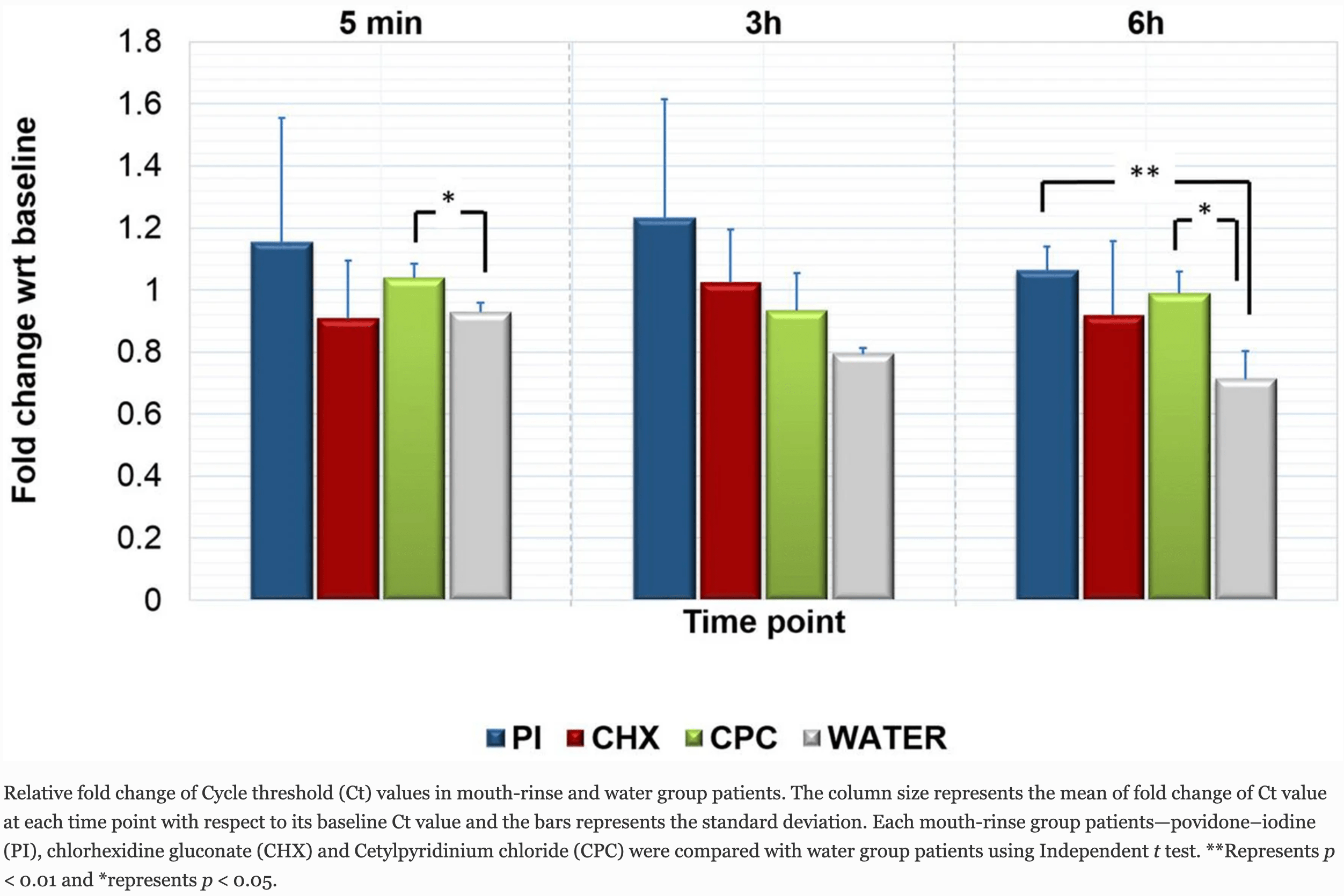
relative fold change, 32.9% better, RR 0.67, p < 0.01, treatment 4, control 2, PVP-I vs. water, 6 hours.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Seneviratne et al., 14 Dec 2020, Randomized Controlled Trial, Singapore, peer-reviewed, 12 authors, study period June 2020 - August 2020.
Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore
Infection, doi:10.1007/s15010-020-01563-9
Purpose One of the key approaches to minimize the risk of COVID-19 transmission would be to reduce the titres of SARS-CoV-2 in the saliva of infected COVID-19 patients. This is particularly important in high-risk procedures like dental treatment. The present randomized control trial evaluated the efficacy of three commercial mouth-rinse viz. povidone-iodine (PI), chlorhexidine gluconate (CHX) and cetylpyridinium chloride (CPC), in reducing the salivary SARS-CoV-2 viral load in COVID-19 patients compared with water. Methods A total of 36 SARS-CoV-2-positive patients were recruited, of which 16 patients were randomly assigned to four groups-PI group (n = 4), CHX group (n = 6), CPC group (n = 4) and water as control group (n = 2). Saliva samples were collected from all patients at baseline and at 5 min, 3 h and 6 h post-application of mouth-rinses/water. The samples were subjected to SARS-CoV-2 RT-PCR analysis. Results Comparison of salivary Ct values of patients within each group of PI, CHX, CPC and water at 5 min, 3 h and 6 h time points did not show any significant differences. However, when the Ct value fold change of each of the mouth-rinse group patients were compared with the fold change of water group patients at the respective time points, a significant increase was observed in the CPC group patients at 5 min and 6 h and in the PI group patients at 6 h.
Conclusion The effect of decreasing salivary load with CPC and PI mouth-rinsing was observed to be sustained at 6 h time point. Within the limitation of the current study, as number of the samples analyzed, the use of CPC and PI formulated that commercial mouth-rinses may be useful as a pre-procedural rinse to help reduce the transmission of COVID-19. ISRCTN (ISRCTN95933274), 09/09/20, retrospectively registered
Author contributions CJS, PB, KKKK, and JSXY contributed to conception, design, data acquisition and interpretation, drafted and critically revised the manuscript. NU, DL, DNHL, IV and GBT contributed to design and data acquisition, drafted and critically revised the manuscript. JLKS, LML, and LO contributed to data acquisition and interpretation, drafted and critically revised the manuscript. All the authors read and approved the final manuscript.
References
Alexander, Gorbalenya, Baric, De Groot, Drosten et al., The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2, Nat microbiol, doi:10.1038/s41564-020-0695-z
Azzi, Carcano, Gianfagna, Grossi, Gasperina et al., Saliva is a reliable tool to detect SARS-CoV-2, J Infect, doi:10.1016/j.jinf.2020.04.005
Bernstein, Schiff, Echler, Prince, Feller et al., In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse, J Dent Res, doi:10.1177/00220345900690030901
Cdc, Interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID-19) pandemic
Chu, Pan, Cheng, Hui, Krishnan et al., Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia, Clin Chem, doi:10.1093/clinchem/hvaa029
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Ge, Yang, Xia, Fu, Zhang, Possible aerosol transmission of COVID-19 and special precautions in dentistry, J Zhejiang Univ Sci B, doi:10.1631/jzus.B2010010
Izzetti, Nisi, Gabriele, Graziani, COVID-19 transmission in dental practice: brief review of preventive measures in Italy, J Dent Res, doi:10.1177/0022034520920580
Joynt, Wu, Understanding COVID-19: what does viral RNA load really mean?, Lancet Infect Dis, doi:10.1016/s1473-3099(20)30237-1
Kawana, Kitamura, Nakagomi, Matsumoto, Arita et al., Inactivation of human viruses by povidoneiodine in comparison with other antiseptics, Dermatology, doi:10.1159/000246027
Lamas, Dios, Rodríguez, Campo, Alvargonzalez et al., Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests Oral Dis, doi:10.1111/odi.13526
Liu, Wei, Alvarez, Wang, Du et al., Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques, J Virol, doi:10.1128/JVI.02292-10
Lu, These are the occupations with the highest COVID-19 risk, World Economic Forum
Meiller, Silva, Ferreira, Jabra-Rizk, Kelley et al., Efficacy of listerine antiseptic in reducing viral contamination of saliva, J Clin Periodontol, doi:10.1111/j.1600-051X.2005.00673.x
Meister, Brüggemann, Todt, Conzelmann, Müller et al., Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J Infect Dis, doi:10.1093/infdis/jiaa471
Meng, Hua, Bian, Coronavirus Disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine, J Dent Res, doi:10.1177/0022034520914246
Mukherjee, Esper, Buchheit, Arters, Adkins et al., Randomized, double-blind, placebo-controlled clinical trial to assess the safety and effectiveness of a novel dualaction oral topical formulation against upper respiratory infections, BMC Infect Dis, doi:10.1186/s12879-016-2177-8
O'donnell, Thomas, Stanton, Maillard, Murphy et al., Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function, doi:10.1093/function/zqaa002
Ott, Strine, Watkins, Boot, Kalinich et al., Simply saliva: stability of SARS-CoV-2 detection negates the need for expensive collection devices, The Preprint Server For Health Sci, doi:10.1101/2020.08.03.20165233
Peng, Xu, Li, Cheng, Zhou et al., Transmission routes of 2019-nCoV and controls in dental practice, Int J Oral Sci, doi:10.1038/s41368-020-0075-9
Popkin, Zilka, Dimaano, Fujioka, Rackley et al., Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathog Immun, doi:10.20411/pai.v2i2.200
Rao, Manissero, Steele, Pareja, A systematic review of the clinical utility of cycle threshold values in the context of COVID-19, Infect Dis The, doi:10.1007/s40121-020-00324-3
To, Tsang, Chik-Yan Yip, Chan, Wu et al., Consistent detection of 2019 novel coronavirus in saliva, Clin Infect Dis, doi:10.1093/cid/ciaa149
Tu, Benn, RRApp, a robust randomization app, for clinical and translational research, J Clin Transl Sci, doi:10.1017/cts.2017.310
Worldometer, Coronavirus Cases
Wu, Chen, Chan, The outbreak of COVID-19: an overview, J Chin Med Assoc, doi:10.1097/JCMA.0000000000000270
Wyllie, Fournier, Casanovas-Massana, Campbell, Tokuyama et al., Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, N Engl J Med, doi:10.1056/NEJMc2016359
Xu, Zhong, Deng, Peng, Dan et al., High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int J Oral Sci, doi:10.1038/s41368-020-0074-x
Yoon, Yoon, Song, Yoon, Lim et al., Clinical significance of a high SARS-CoV-2 viral load in the saliva, J Korean Med Sci, doi:10.3346/jkms.2020.35.e195
Young, Fong, Chan, Mak, Ang et al., Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study, Lancet, doi:10.1016/s0140-6736(20)31757-8
DOI record:
{
"DOI": "10.1007/s15010-020-01563-9",
"ISSN": [
"0300-8126",
"1439-0973"
],
"URL": "http://dx.doi.org/10.1007/s15010-020-01563-9",
"alternative-id": [
"1563"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "25 September 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "24 November 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "14 December 2020"
},
{
"group": {
"label": "Compliance with ethical standards",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article."
},
{
"group": {
"label": "Ethics approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Ethics approval was obtained from SingHealth Centralized Institutional Review Board (CIRB Ref No: 2020/2537).Consent to participate. All the patients provided informed consent upon recruitment in the study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "All the authors have provided consent for publication."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"affiliation": [],
"family": "Seneviratne",
"given": "Chaminda J.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Balan",
"given": "Preethi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ko",
"given": "Kwan Ki Karrie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Udawatte",
"given": "Nadeeka S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lai",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ng",
"given": "Dorothy Hui Lin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Venkatachalam",
"given": "Indumathi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Kheng Sit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ling",
"given": "Moi Lin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oon",
"given": "Lynette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goh",
"given": "Bee Tin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sim",
"given": "Xiang Ying Jean",
"sequence": "additional"
}
],
"container-title": "Infection",
"container-title-short": "Infection",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
12,
14
]
],
"date-time": "2020-12-14T07:03:21Z",
"timestamp": 1607929401000
},
"deposited": {
"date-parts": [
[
2021,
3,
24
]
],
"date-time": "2021-03-24T09:29:22Z",
"timestamp": 1616578162000
},
"funder": [
{
"award": [
"(133/20)",
"(11/FY2019/G1/02-A44)"
],
"name": "NDCS/NDRIS"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T09:10:10Z",
"timestamp": 1712221810729
},
"is-referenced-by-count": 120,
"issue": "2",
"issued": {
"date-parts": [
[
2020,
12,
14
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
14
]
],
"date-time": "2020-12-14T00:00:00Z",
"timestamp": 1607904000000
}
},
{
"URL": "http://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
14
]
],
"date-time": "2020-12-14T00:00:00Z",
"timestamp": 1607904000000
}
}
],
"link": [
{
"URL": "http://link.springer.com/content/pdf/10.1007/s15010-020-01563-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/article/10.1007/s15010-020-01563-9/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/content/pdf/10.1007/s15010-020-01563-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "305-311",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2020,
12,
14
]
]
},
"published-online": {
"date-parts": [
[
2020,
12,
14
]
]
},
"published-print": {
"date-parts": [
[
2021,
4
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "1563_CR1",
"unstructured": "Worldometer. Coronavirus Cases. 2020. https://www.worldometers.info/coronavirus/coronavirus-cases/?fbclid=IwAR2w6eAL-6QTY0lqv-KTr2ka1Y__t7d720WfKoOfEA9O-ZpVfRWWEhEskfI. Accessed August 31 2020."
},
{
"DOI": "10.1097/JCMA.0000000000000270",
"author": "Y-C Wu",
"doi-asserted-by": "publisher",
"first-page": "217",
"journal-title": "J Chin Med Assoc",
"key": "1563_CR2",
"unstructured": "Wu Y-C, Chen C-S, Chan Y-J. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83:217–20. https://doi.org/10.1097/JCMA.0000000000000270.",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa149",
"author": "KK-W To",
"doi-asserted-by": "publisher",
"first-page": "841",
"journal-title": "Clin Infect Dis",
"key": "1563_CR3",
"unstructured": "To KK-W, Tsang OT-Y, Chik-Yan Yip C, Chan K-H, Wu T-C, Chan JMC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–3. https://doi.org/10.1093/cid/ciaa149.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.03.20165233",
"author": "IM Ott",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv: The Preprint Server For Health Sci",
"key": "1563_CR4",
"unstructured": "Ott IM, Strine MS, Watkins AE, Boot M, Kalinich CC, Harden CA, et al. Simply saliva: stability of SARS-CoV-2 detection negates the need for expensive collection devices. medRxiv: The Preprint Server For Health Sci. 2020. https://doi.org/10.1101/2020.08.03.20165233.",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2016359",
"author": "AL Wyllie",
"doi-asserted-by": "publisher",
"first-page": "1283",
"journal-title": "N Engl J Med",
"key": "1563_CR5",
"unstructured": "Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–6. https://doi.org/10.1056/NEJMc2016359.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1177/0022034520914246",
"author": "L Meng",
"doi-asserted-by": "publisher",
"first-page": "481",
"journal-title": "J Dent Res",
"key": "1563_CR6",
"unstructured": "Meng L, Hua F, Bian Z. Coronavirus Disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99:481–7. https://doi.org/10.1177/0022034520914246.",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1177/0022034520920580",
"author": "R Izzetti",
"doi-asserted-by": "publisher",
"first-page": "1030",
"journal-title": "J Dent Res",
"key": "1563_CR7",
"unstructured": "Izzetti R, Nisi M, Gabriele M, Graziani F. COVID-19 transmission in dental practice: brief review of preventive measures in Italy. J Dent Res. 2020;99:1030–8. https://doi.org/10.1177/0022034520920580.",
"volume": "99",
"year": "2020"
},
{
"key": "1563_CR8",
"unstructured": "Lu M. These are the occupations with the highest COVID-19 risk. In: World Economic Forum. 20 April 2020. https://www.weforum.org/agenda/2020/04/occupations-highest-covid19-risk/."
},
{
"DOI": "10.1038/s41368-020-0075-9",
"author": "X Peng",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Int J Oral Sci",
"key": "1563_CR9",
"unstructured": "Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12:9. https://doi.org/10.1038/s41368-020-0075-9.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"author": "JG Yoon",
"doi-asserted-by": "publisher",
"first-page": "e195",
"journal-title": "J Korean Med Sci",
"key": "1563_CR10",
"unstructured": "Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35:e195. https://doi.org/10.3346/jkms.2020.35.e195.",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1111/odi.13526",
"author": "L Martínez Lamas",
"doi-asserted-by": "publisher",
"journal-title": "First in vivo tests Oral Dis",
"key": "1563_CR11",
"unstructured": "Martínez Lamas L, Diz Dios P, Pérez Rodríguez MT, Del Campo PV, Cabrera Alvargonzalez JJ, López Domínguez AM, et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests Oral Dis. 2020. https://doi.org/10.1111/odi.13526.",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa471",
"author": "TL Meister",
"doi-asserted-by": "publisher",
"first-page": "1289",
"journal-title": "J Infect Dis",
"key": "1563_CR12",
"unstructured": "Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, Groß R, et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222:1289–92. https://doi.org/10.1093/infdis/jiaa471.",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1017/cts.2017.310",
"author": "C Tu",
"doi-asserted-by": "publisher",
"first-page": "323",
"journal-title": "J Clin Transl Sci",
"key": "1563_CR13",
"unstructured": "Tu C, Benn EKT. RRApp, a robust randomization app, for clinical and translational research. J Clin Transl Sci. 2017;1:323–7. https://doi.org/10.1017/cts.2017.310.",
"volume": "1",
"year": "2017"
},
{
"DOI": "10.1016/j.jinf.2020.04.005",
"author": "L Azzi",
"doi-asserted-by": "publisher",
"first-page": "e45",
"journal-title": "J Infect",
"key": "1563_CR14",
"unstructured": "Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina DD, Genoni A, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81:e45–50. https://doi.org/10.1016/j.jinf.2020.04.005.",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"author": "VM Corman",
"doi-asserted-by": "publisher",
"first-page": "2000045",
"journal-title": "Euro Surveill",
"key": "1563_CR15",
"unstructured": "Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045.",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1093/clinchem/hvaa029",
"author": "DKW Chu",
"doi-asserted-by": "publisher",
"first-page": "549",
"journal-title": "Clin Chem",
"key": "1563_CR16",
"unstructured": "Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–55. https://doi.org/10.1093/clinchem/hvaa029.",
"volume": "66",
"year": "2020"
},
{
"DOI": "10.1007/s40121-020-00324-3",
"author": "SN Rao",
"doi-asserted-by": "publisher",
"first-page": "573",
"journal-title": "Infect Dis The",
"key": "1563_CR17",
"unstructured": "Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis The. 2020;9:573–86. https://doi.org/10.1007/s40121-020-00324-3.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1111/j.1600-051X.2005.00673.x",
"author": "TF Meiller",
"doi-asserted-by": "publisher",
"first-page": "341",
"journal-title": "J Clin Periodontol",
"key": "1563_CR18",
"unstructured": "Meiller TF, Silva A, Ferreira SM, Jabra-Rizk MA, Kelley JI, DePaola LG. Efficacy of listerine antiseptic in reducing viral contamination of saliva. J Clin Periodontol. 2005;32:341–6. https://doi.org/10.1111/j.1600-051X.2005.00673.x.",
"volume": "32",
"year": "2005"
},
{
"DOI": "10.1631/jzus.B2010010",
"author": "Z-Y Ge",
"doi-asserted-by": "publisher",
"first-page": "361",
"journal-title": "J Zhejiang Univ Sci B",
"key": "1563_CR19",
"unstructured": "Ge Z-Y, Yang L-M, Xia J-J, Fu X-H, Zhang Y-Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 2020;21:361–8. https://doi.org/10.1631/jzus.B2010010.",
"volume": "21",
"year": "2020"
},
{
"key": "1563_CR20",
"unstructured": "CDC. Guidance for dental settings. Interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID-19) pandemic. In: Healthcare Workers. Center for Disease Control and Prevention 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html. Accessed 29 August 2020."
},
{
"key": "1563_CR21",
"unstructured": "ADA. Australian Dental Association, ADA COVID-19 Risk management guidance. 2020. https://www.ada.org.au/Covid-19-Portal/Files/pdf/COVID-19-Risk-Management-Guidance.aspx. Last updated 4 August 2020."
},
{
"DOI": "10.20411/pai.v2i2.200",
"author": "DL Popkin",
"doi-asserted-by": "publisher",
"first-page": "252",
"journal-title": "Pathog Immun",
"key": "1563_CR22",
"unstructured": "Popkin DL, Zilka S, Dimaano M, Fujioka H, Rackley C, Salata R, et al. Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo. Pathog Immun. 2017;2:252–69. https://doi.org/10.20411/pai.v2i2.200.",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1159/000246027",
"author": "R Kawana",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Dermatology (Basel, Switzerland)",
"key": "1563_CR23",
"unstructured": "Kawana R, Kitamura T, Nakagomi O, Matsumoto I, Arita M, Yoshihara N, et al. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology (Basel, Switzerland). 1997;195:29–35. https://doi.org/10.1159/000246027.",
"volume": "195",
"year": "1997"
},
{
"DOI": "10.1177/00220345900690030901",
"author": "D Bernstein",
"doi-asserted-by": "publisher",
"first-page": "874",
"journal-title": "J Dent Res",
"key": "1563_CR24",
"unstructured": "Bernstein D, Schiff G, Echler G, Prince A, Feller M, Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J Dent Res. 1990;69:874–6. https://doi.org/10.1177/00220345900690030901.",
"volume": "69",
"year": "1990"
},
{
"DOI": "10.1093/function/zqaa002",
"author": "VB O’Donnell",
"doi-asserted-by": "publisher",
"journal-title": "Function",
"key": "1563_CR25",
"unstructured": "O’Donnell VB, Thomas D, Stanton R, Maillard J-Y, Murphy RC, Jones SA, et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020. https://doi.org/10.1093/function/zqaa002.",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"author": "E Alexander",
"doi-asserted-by": "publisher",
"first-page": "536",
"journal-title": "Nat microbiol",
"key": "1563_CR26",
"unstructured": "Alexander E, Gorbalenya SCB, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat microbiol. 2020;5:536–44. https://doi.org/10.1038/s41564-020-0695-z.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1186/s12879-016-2177-8",
"author": "PK Mukherjee",
"doi-asserted-by": "publisher",
"first-page": "74",
"journal-title": "BMC Infect Dis",
"key": "1563_CR27",
"unstructured": "Mukherjee PK, Esper F, Buchheit K, Arters K, Adkins I, Ghannoum MA, et al. Randomized, double-blind, placebo-controlled clinical trial to assess the safety and effectiveness of a novel dual-action oral topical formulation against upper respiratory infections. BMC Infect Dis. 2017;17:74. https://doi.org/10.1186/s12879-016-2177-8.",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1128/JVI.02292-10",
"author": "L Liu",
"doi-asserted-by": "publisher",
"first-page": "4025",
"journal-title": "J Virol",
"key": "1563_CR28",
"unstructured": "Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85:4025–30. https://doi.org/10.1128/JVI.02292-10.",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"author": "H Xu",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Int J Oral Sci",
"key": "1563_CR29",
"unstructured": "Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. https://doi.org/10.1038/s41368-020-0074-x.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/s0140-6736(20)31757-8",
"author": "BE Young",
"doi-asserted-by": "publisher",
"first-page": "603",
"journal-title": "Lancet (London, England)",
"key": "1563_CR30",
"unstructured": "Young BE, Fong SW, Chan YH, Mak TM, Ang LW, Anderson DE, et al. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet (London, England). 2020;396:603–11. https://doi.org/10.1016/s0140-6736(20)31757-8.",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1016/s1473-3099(20)30237-1",
"author": "GM Joynt",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "1563_CR31",
"unstructured": "Joynt GM, Wu WK. Understanding COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020. https://doi.org/10.1016/s1473-3099(20)30237-1.",
"year": "2020"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "http://link.springer.com/10.1007/s15010-020-01563-9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "49"
}
