Favipiravir Exposure in the Second and Third Trimesters of Pregnancy with COVID-19: Risk of Adverse Perinatal Outcomes: A Retrospective Cohort Study
et al., Journal of the Medical Association of Thailand, doi:10.35755/jmedassocthai.2025.6.431-439-01780, Jun 2025
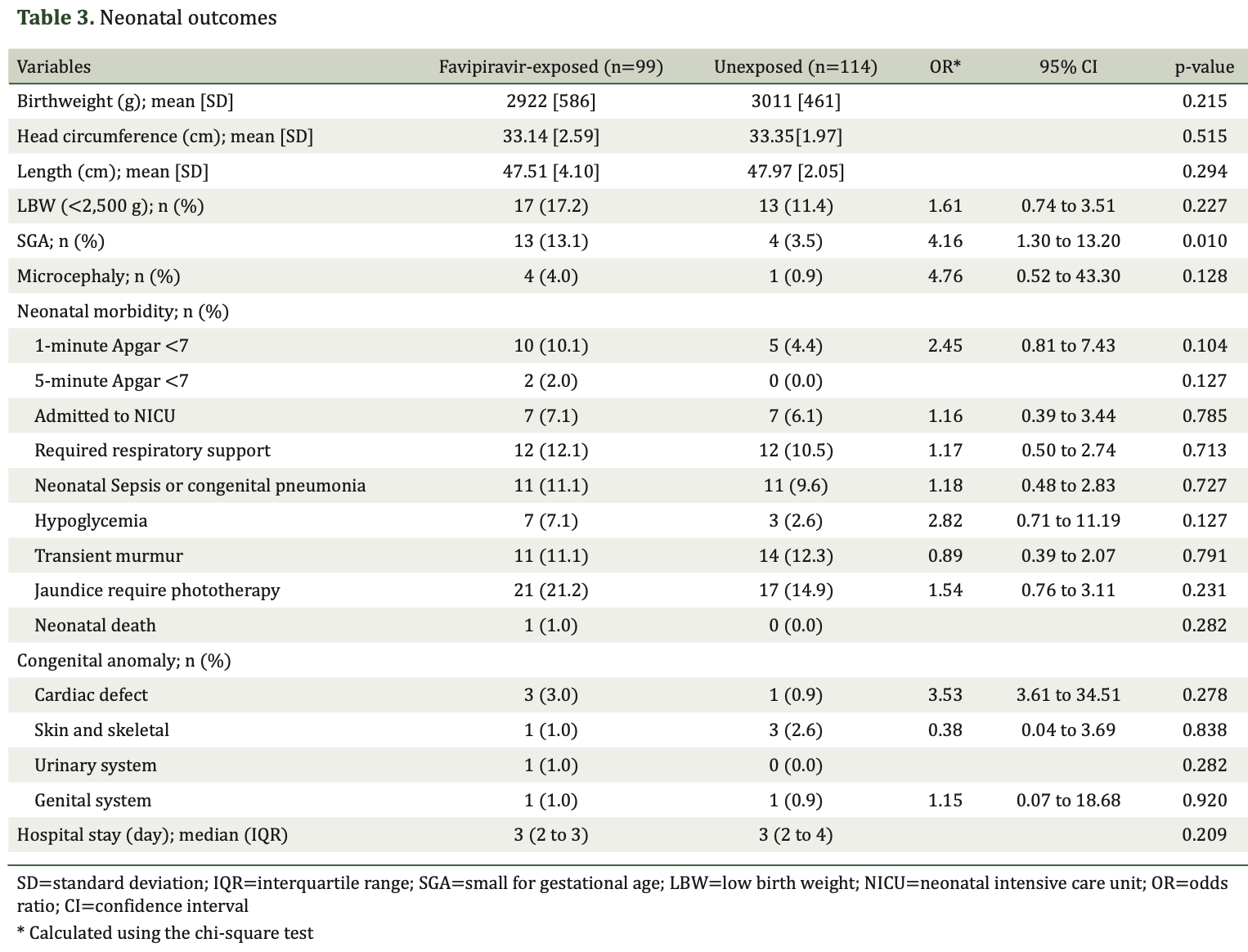
Retrospective 214 pregnant women with COVID-19 showing increased risk of small for gestational age (SGA) infants with favipiravir exposure during the second and third trimesters.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Noomcharoen et al., 23 Jun 2025, retrospective, Thailand, peer-reviewed, 2 authors, study period 1 June, 2021 - 31 May, 2022.
Contact: onanong@somdej-mec.or.th.
Favipiravir Exposure in the Second and Third Trimesters of Pregnancy with COVID-19: Risk of Adverse Perinatal Outcomes: A Retrospective Cohort Study
Journal of the Medical Association of Thailand, doi:10.35755/jmedassocthai.2025.6.431-439-01780
Background: During the pandemic, Favipiravir, an oral antiviral agent, was used for COVID-19 treatment. However, its safety profile for pregnant women, especially during the second and third trimesters, is not well-established. Objective: To assess the pregnancy outcomes and risk of congenital anomalies associated with favipiravir exposure during the second and third trimesters in COVID-19-infected pregnant women.
Materials and Methods: A retrospective cohort study was conducted at the Department of Obstetrics and Gynecology, Queen Savang Vadhana Memorial Hospital, including pregnant women beyond 14 weeks gestation who delivered between June 1, 2021, and May 31, 2022. Participants were divided into those exposed to favipiravir, which were 99 patients, and those who were not exposed, which included 115 patients. Data on maternal, obstetric, and neonatal outcomes were collected and analyzed. Results: Out of 383 registered COVID-19-infected pregnant women, 214 were included in the analysis. No severe maternal drug reactions were observed. Favipiravir exposure was significantly associated with an increased rate of small for gestational age (SGA) infants at 13.1% versus 3.5% (OR 4.16, p=0.01). No significant differences were found in other obstetric and neonatal outcomes, including preterm delivery, cesarean delivery, or neonatal morbidity. Specific congenital anomalies such as cardiac defects and renal pyelectasis were observed in the favipiravir-exposed group, but these were not statistically significant.
Conclusion: Favipiravir exposure during the second and third trimesters was associated with an increased risk of SGA, while congenital anomalies and other perinatal outcomes did not differ significantly between groups.
Conflicts of interest The authors have no conflicts of interest.
References
Arco-Torres, Cortés-Martín, Tovar-Gálvez, Montiel-Troya, Riquelme-Gallego et al., Pharmacological treatments against COVID-19 in pregnant women, J Clin Med, doi:10.3390/jcm10214896
Balasubramanian, Vuppalapati, Avanthika, Jhaveri, Peddi et al., Epidemiology, genetics and epigenetics of congenital heart diseases in twins, Cureus
Batool, Vuthaluru, Hassan, Bseiso, Tehseen et al., Efficacy and Safety of Favipiravir in Treating COVID-19 Patients: A Meta-Analysis of Randomized Control Trials, Cureus
Bilir, Atay, Firat, Kundakci, Investigation of developmental toxicity of favipiravir on fetal bone and embryonic development, Birth Defects Res
Black, Global prevalence of small for gestational age births, Nestle Nutr Inst Workshop Ser
Boriboonhirunsarn, Srikureja, Rates of small for gestational age and low birth weight among underweight and normal weight women, Minerva Obstet Gynecol
Caluwaerts, Nubia's mother: being pregnant in the time of experimental vaccines and therapeutics for Ebola, Reprod Health, doi:10.1186/s12978-017-0429-8
Demir, Albayrak, Aslan, The impact of coronavirus disease-19 on pregnancy outcomes, a case series, Gynecol Obstet Reprod Med
Deng, Yang, Yang, Chen, Qiu et al., Evaluation of favipiravir in the treatment of COVID-19 based on the real-world, Expert Rev Anti Infect Ther
Ertem, Guner, Incir, Kalkan, Gelal, The outcomes of favipiravir exposure in pregnancy: a case series, Arch Gynecol Obstet
Gennaro, Guido, Frallonardo, Segala, Nola et al., Efficacy and safety of therapies for COVID-19 in pregnancy: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-023-08747-2
Giesbers, Goh, Kew, Allotey, Brizuela et al., Treatment of COVID-19 in pregnant women: A systematic review and meta-analysis, Eur J Obstet Gynecol Reprod Biol
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Hoover, Yamamura, Thompson, Structural anomalies in multifetal gestations, Clin Obstet Gynecol
Jafari, Jonaidi-Jafari, Dehghanpoor, Saburi, Convalescent plasma therapy in a pregnant COVID-19 patient with a dramatic clinical and imaging response: A case report, World J Radiol
Korula, Alexander, John, Kirubakaran, Singh et al., Favipiravir for treating COVID-19, Cochrane Database Syst Rev
Laçin, Turhan, Güngördü, Assessing the impact of antiviral drugs commonly utilized during the COVID-19 pandemic on the embryonic development of Xenopus laevis, J Hazard Mater, doi:10.1016/j.jhazmat.2024.134462
Lin, Liang, Chuang, Tseng, Tsai et al., Clinical outcomes of nirmatrelvir-ritonavir use in pregnant women during the Omicron wave of the coronavirus disease 2019 pandemic, J Infect Public Health
Louchet, Sibiude, Peytavin, Picone, Tréluyer et al., Placental transfer and safety in pregnancy of medications under investigation to treat coronavirus disease 2019, Am J Obstet Gynecol MFM, doi:10.1016/j.ajogmf.2020.100159
Martínez, Murillo, Mora, Mereles, Tetralogy of Fallot: Hypoxia, the villain of the story?, Birth Defects Res
Nana, Hodson, Lucas, Camporota, Knight et al., Diagnosis and management of covid-19 in pregnancy, BMJ
Osuchukwu, Reed, Small for gestational age
Pairat, Phaloprakarn, Acceptance of COVID-19 vaccination during pregnancy among Thai pregnant women and their spouses: a prospective survey, Reprod Health, doi:10.1186/s12978-022-01383-0
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, J Virus Erad
Rattanaumpawan, Jirajariyavej, Lerdlamyong, Palavutitotai, Saiyarin, Real-world effectiveness and optimal dosage of favipiravir for treatment of COVID-19: Results from a multicenter observational study in Thailand, Antibiotics, doi:10.3390/antibiotics11060805
Siripongboonsitti, Tawinprai, Cheirsilpa, Ungtrakul, Krisorakun et al., The real-world clinical outcomes of favipiravir treatment with telemedicine monitoring in preventing disease progression in mild to moderate COVID-19 patients; a retrospective cohort study, Medicina, doi:10.3390/medicina59061098
Surapat, Kobpetchyok, Kiertiburanakul, Arnuntasupakul, Use of favipiravir for the treatment of coronavirus disease 2019 in the setting of hospitel, Int J Clin Pract, doi:10.1155/2022/3098527
Tırmıkçıoğlu, Favipiravir exposure and pregnancy outcome of COVID-19 patients, Eur J Obstet Gynecol Reprod Biol
Wong, Lau, Chung, Au, Cheung et al., Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation, Nat Med
Özen, Us, Toplu, Vizdiklar, Selalmaz et al., Favipiravir does not appear to be a major teratogen: Case series from Türkiye, J Gynecol Obstet Hum Reprod, doi:10.1016/j.jogoh.2023.102693
Özlüşen, Kozan, Akcan, Kalender, Yaprak et al., Effectiveness of favipiravir in COVID-19: a live systematic review, Eur J Clin Microbiol Infect Dis
DOI record:
{
"DOI": "10.35755/jmedassocthai.2025.6.431-439-01780",
"ISSN": [
"2408-1981",
"0125-2208"
],
"URL": "http://dx.doi.org/10.35755/jmedassocthai.2025.6.431-439-01780",
"abstract": "<jats:p>Background: During the pandemic, Favipiravir, an oral antiviral agent, was used for COVID-19 treatment. However, its safety profile for pregnant women, especially during the second and third trimesters, is not well-established.\nObjective: To assess the pregnancy outcomes and risk of congenital anomalies associated with favipiravir exposure during the second and third trimesters in COVID-19-infected pregnant women.\nMaterials and Methods: A retrospective cohort study was conducted at the Department of Obstetrics and Gynecology, Queen Savang Vadhana Memorial Hospital, including pregnant women beyond 14 weeks gestation who delivered between June 1, 2021, and May 31, 2022. Participants were divided into those exposed to favipiravir, which were 99 patients, and those who were not exposed, which included 115 patients. Data on maternal, obstetric, and neonatal outcomes were collected and analyzed.\nResults: Out of 383 registered COVID-19-infected pregnant women, 214 were included in the analysis. No severe maternal drug reactions were observed. Favipiravir exposure was significantly associated with an increased rate of small for gestational age (SGA) infants at 13.1% versus 3.5% (OR 4.16, p=0.01). No significant differences were found in other obstetric and neonatal outcomes, including preterm delivery, cesarean delivery, or neonatal morbidity. Specific congenital anomalies such as cardiac defects and renal pyelectasis were observed in the favipiravir-exposed group, but these were not statistically significant.\nConclusion: Favipiravir exposure during the second and third trimesters was associated with an increased risk of SGA, while congenital anomalies and other perinatal outcomes did not differ significantly between groups.</jats:p>",
"container-title": "Journal of the Medical Association of Thailand",
"container-title-short": "J Med Assoc Thai",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T04:47:11Z",
"timestamp": 1750740431000
},
"deposited": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T04:47:12Z",
"timestamp": 1750740432000
},
"indexed": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T05:10:11Z",
"timestamp": 1750741811051,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2025,
6,
23
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2025,
6,
23
]
]
}
},
"language": "en",
"member": "20641",
"original-title": [],
"page": "431-439",
"prefix": "10.35755",
"published": {
"date-parts": [
[
2025,
6,
23
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
23
]
]
},
"publisher": "Medical Association of Thailand",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.jmatonline.com/view.php?id=8495"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Favipiravir Exposure in the Second and Third Trimesters of Pregnancy with COVID-19: Risk of Adverse Perinatal Outcomes: A Retrospective Cohort Study",
"type": "journal-article",
"volume": "108"
}