Clinical outcome, viral response and safety profile of chloroquine in COVID-19 patients — initial experience
et al., Advances in Respiratory Medicine, doi:10.5603/ARM.a2020.0139, Nov 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
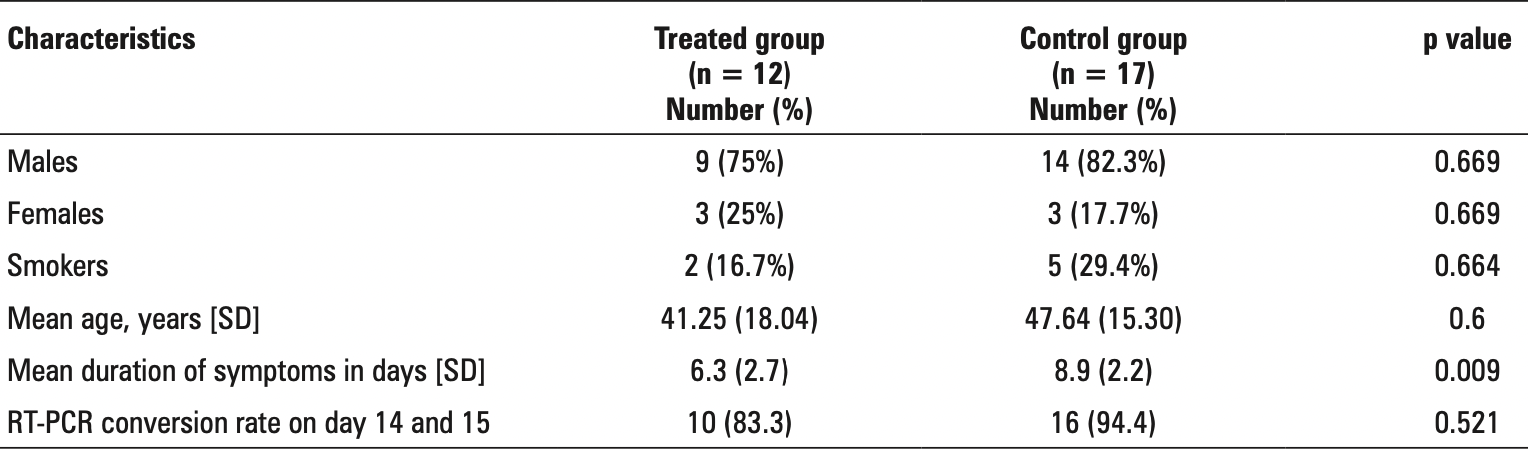
Retrospective 12 hospitalized patients in India treated with CQ and 17 controls, showing faster recovery with treatment. There was no significant difference in viral clearance. The CQ group mean age was 41.3 vs. 47.6 for controls.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
This study is excluded in the after exclusion results of meta-analysis:
excessive unadjusted differences between groups.
|
recovery time, 29.2% lower, relative time 0.71, p = 0.008, treatment mean 6.3 (±2.7) n=12, control mean 8.9 (±2.2) n=17.
|
|
risk of no viral clearance, 183.3% higher, RR 2.83, p = 0.55, treatment 2 of 12 (16.7%), control 1 of 17 (5.9%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Niwas et al., 1 Nov 2020, retrospective, India, peer-reviewed, mean age 45.5, 17 authors.
Contact: rniwasaiims@gmail.com.
Clinical Outcome, Viral Response and Safety Profile of Chloroquine in COVID-19 Patients—Initial Experience
Advances in Respiratory Medicine, doi:10.5603/arm.a2020.0139
Introduction: Chloroquine and its analogues are currently being investigated for the treatment and post exposure prophylaxis of COVID-19 due to its antiviral activity and immunomodulatory activity. Material and methods: Confirmed symptomatic cases of COVID-19 were included in the study. Patients were supposed to receive chloroquine (CQ) 500 mg twice daily for 7 days. Due to a change in institutional protocol, initial patients received chloroquine and subsequent patients who did not receive chloroquine served as negative controls. Clinical effectiveness was determined in terms of timing of symptom resolution and conversion rate of reverse transcriptase polymerase chain reaction (RT-PCR) on day 14 and day 15 of admission. Results: Twelve COVID-19 patients formed the treatment arm and 17 patients were included in the control arm. The duration of symptoms among the CQ treated group (6.3 ± 2.7 days) was significantly (p-value = 0.009) lower than that of the control group (8.9 ± 2.2 days). There was no significant difference in the rate of RT-PCR negativity in both groups. 2 patients out of 12 developed diarrhea in the CQ therapy arm.
Conclusion: The duration of symptoms among the treated group (with chloroquine) was significantly lower than that of the control group. RT-PCR conversion was not significantly different between the 2 groups.
Conflict of interest None declared.
References
Agostini, Andres, Sims, Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, mBio, doi:10.1128/mBio.00221-18
Gao, Tian, Xu, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends, doi:10.5582/bst.2020.01047
Guan, Ni, Yu, Clinical characteristics of coronavirus disease 2019 in china, N Engl J Med, doi:10.1056/NEJMoa2002032
Jie, He, Xi, Zhi, Multicenter Collaboration Group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumoni-aExpert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia, Chinese
Liu, Xiao, Wei, Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2, J Med Virol, doi:10.1002/jmv.25726
Liu, Zhang, Huang, -novel coronavirus (2019-nCoV) infections trigger an exaggerated cytokine response aggravating lung injury
Lu, Zhao, Li, Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet, doi:10.1016/S0140-6736(20)30251-8
Na, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
Rosendaal, Gautret, Lagier, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105949
Savarino, Ldi, Donatelli, New insights into the antiviral effects of chloroquine, The Lancet Infectious Diseas, doi:10.1016/s1473-3099(06)70361-9
Tang, Cao, Han, Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial, BMJ, doi:10.1136/bmj.m1849
Van Den Broek, Möhlmann, Abeln, Chloroquine-induced QTc prolongation in COVID-19 patients, Neth Heart J, doi:10.1007/s12471-020-01429-7
Wahba, Jain, Fire, A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Who, Coronavirus disease (COVID-2019) situation reports
Zumla, Chan, Azhar, Coronaviruses -drug discovery and therapeutic options, Nat Rev Drug Discov, doi:10.1038/nrd.2015.37
DOI record:
{
"DOI": "10.5603/arm.a2020.0139",
"ISSN": [
"2543-6031",
"2451-4934"
],
"URL": "http://dx.doi.org/10.5603/arm.a2020.0139",
"author": [
{
"affiliation": [],
"family": "Niwas",
"given": "Ram",
"sequence": "first"
},
{
"affiliation": [],
"family": "S",
"given": "Aneesa Shahul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garg",
"given": "M K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nag",
"given": "Vijaya Lakshmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhatia",
"given": "Pradeep Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dutt",
"given": "Naveen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chauhan",
"given": "Nishant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charan",
"given": "Jaykaran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asfahan",
"given": "Shahir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Praveen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhardwaj",
"given": "Pankaj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banerjee",
"given": "Mithu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garg",
"given": "Pawan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sureka",
"given": "Binit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bohra",
"given": "Gopal Krishna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gopalakrishnan",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Misra",
"given": "Sanjeev",
"sequence": "additional"
}
],
"container-title": [
"Advances in Respiratory Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
1,
4
]
],
"date-time": "2021-01-04T12:27:53Z",
"timestamp": 1609763273000
},
"deposited": {
"date-parts": [
[
2021,
1,
4
]
],
"date-time": "2021-01-04T12:27:58Z",
"timestamp": 1609763278000
},
"indexed": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T17:13:32Z",
"timestamp": 1648919612166
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "electronic",
"value": "2543-6031"
},
{
"type": "print",
"value": "2451-4934"
}
],
"issue": "6",
"issued": {
"date-parts": [
[
2020,
12,
30
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2020,
12,
30
]
]
}
},
"link": [
{
"URL": "https://journals.viamedica.pl/advances_in_respiratory_medicine/article/viewFile/69692/52404",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3595",
"original-title": [],
"page": "515-519",
"prefix": "10.5603",
"published": {
"date-parts": [
[
2020,
12,
30
]
]
},
"published-online": {
"date-parts": [
[
2020,
12,
30
]
]
},
"publisher": "VM Media SP. zo.o VM Group SK",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.viamedica.pl/advances_in_respiratory_medicine/article/view/69692"
}
},
"score": 1,
"short-container-title": [
"Adv Respir Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": [
"Clinical outcome, viral response and safety profile of chloroquine in COVID-19 patients — initial experience"
],
"type": "journal-article",
"volume": "88"
}
