Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.00000015 from 57 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,300+ studies for
210+ treatments. c19early.org
|
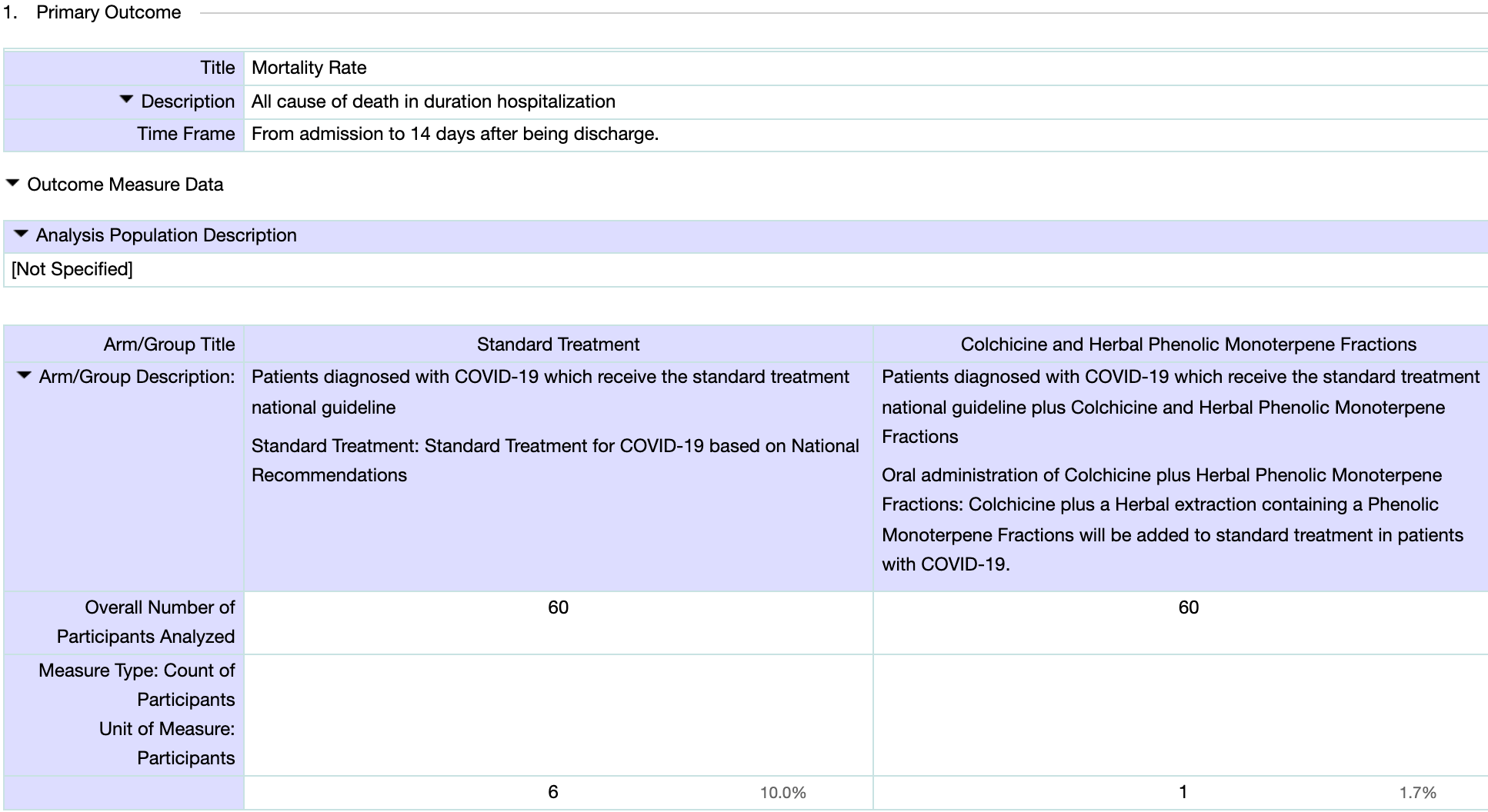
RCT with 60 patients treated with colchicine and phenolic monoterpenes and 60 control patients in Iran, showing lower mortality with treatment. NCT04392141 (history).
|
risk of death, 83.3% lower, RR 0.17, p = 0.11, treatment 1 of 60 (1.7%), control 6 of 60 (10.0%), NNT 12, primary outcome.
|
|
hospitalization time, 34.7% lower, relative time 0.65, p < 0.001, treatment 59, control 54.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mostafaie et al., 20 Apr 2021, Randomized Controlled Trial, Iran, preprint, 1 author, study period 1 April, 2020 - 1 November, 2020, dosage not specified, this trial uses multiple treatments in the treatment arm (combined with phenolic monoterpenes) - results of individual treatments may vary, trial NCT04392141 (history).