Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi
et al., Circulation, doi:10.1161/CIRCULATIONAHA.117.027290, Aug 2017
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
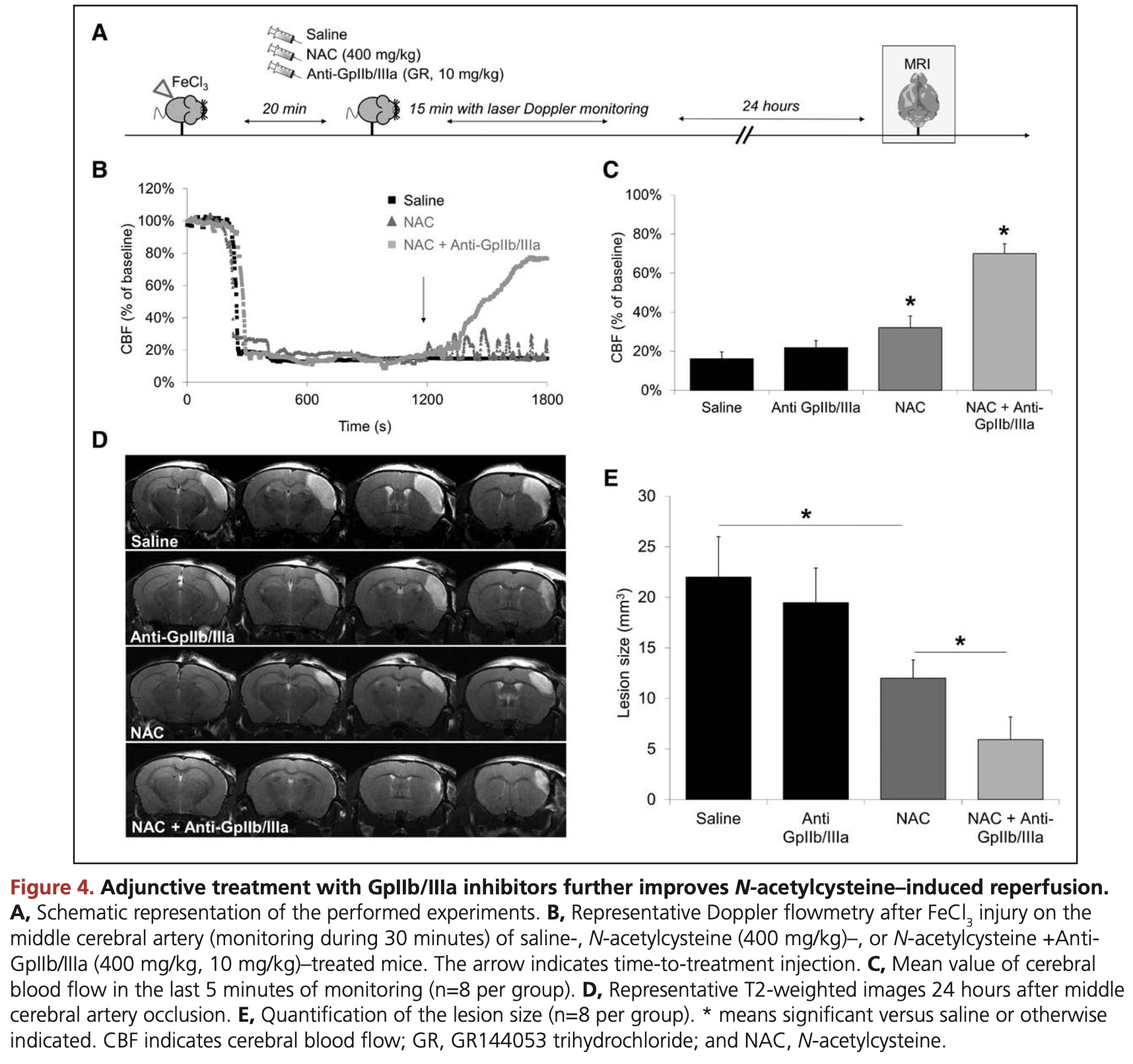
Experimental study in mice showing potent thrombolytic effects with N-acetylcysteine (NAC) in arterial thrombosis models. Authors hypothesize that NAC's thrombolytic mechanism involves destabilizing the VWF-dependent outer portion of thrombi, exposing the inner core to GpIIb/IIIa inhibition.
14 preclinical studies support the efficacy of N-acetylcysteine for COVID-19:
Severe COVID-19 is marked by endotheliopathy with elevated von Willebrand factor (VWF) levels and platelet/VWF-rich microthrombi; N-acetylcysteine can reduce VWF multimers and lyse VWF-dependent clots in vivo, potentially helping to alleviate thrombosis associated with COVID-1910-12.
N-acetylcysteine shows dose-dependent inhibition of SARS-CoV-24,7,9 , shows anti-inflammatory and immunomodulatory effects against SARS-CoV-2-induced immune responses in combination with bromelain6, suppressed virus-induced reactive oxygen species and blocked viral replication in a humanized mouse model and in human lung cells5, may limit COVID-19 induced cardiac damage by boosting cellular antioxidant defenses and potentially mitigating the oxidative stress caused by spike protein-induced ROS production in cardiac fibroblasts3, and reduces disulfide bonds in proteins and exhibits antioxidant properties that may inhibit viral replication and modulate inflammatory responses2.
NAC may be beneficial for COVID-19 by replenishing glutathione stores and reinforcing the glutathione peroxidase-4 pathway to inhibit ferroptosis, an oxidative stress-induced cell death pathway implicated in COVID-1913.
NAC reinforces glutathione levels, reduces ROS, and minimizes ferroptosis and cytokine storm14.
1.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
2.
Reis et al., Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant, Scientific Reports, doi:10.1038/s41598-025-92242-y.
3.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
4.
Chaopreecha et al., Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC), Phytomedicine, doi:10.1016/j.phymed.2024.156279.
5.
Frasson et al., Identification of druggable host dependency factors shared by multiple SARS-CoV-2 variants of concern, Journal of Molecular Cell Biology. doi:10.1093/jmcb/mjae004, academic.oup.com/jmcb/advance-article/doi/10.1093/jmcb/mjae004/7596546.
6.
Ferreira et al., Taming the SARS-CoV-2-mediated proinflammatory response with BromAc®, Frontiers in Immunology, doi:10.3389/fimmu.2023.1308477.
7.
La Maestra et al., Inhibition of the Cell Uptake of Delta and Omicron SARS-CoV-2 Pseudoviruses by N-Acetylcysteine Irrespective of the Oxidoreductive Environment, Cells, doi:10.3390/cells11203313.
8.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
9.
Akhter et al., The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2, Viruses, doi:10.3390/v13030425.
10.
Martinez de Lizarrondo et al., Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi, Circulation, doi:10.1161/CIRCULATIONAHA.117.027290.
11.
Chen et al., N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice, Journal of Clinical Investigation, doi:10.1172/JCI41062.
12.
Goshua et al., Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study, The Lancet Haematology, doi:10.1016/S2352-3026(20)30216-7.
Martinez de Lizarrondo et al., 15 Aug 2017, peer-reviewed, 11 authors.
Contact: gauberti@cyceron.fr.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Potent Thrombolytic Effect of N -Acetylcysteine on Arterial Thrombi
Circulation, doi:10.1161/circulationaha.117.027290
BACKGROUND: Platelet cross-linking during arterial thrombosis involves von Willebrand Factor (VWF) multimers. Therefore, proteolysis of VWF appears promising to disaggregate platelet-rich thrombi and restore vessel patency in acute thrombotic disorders such as ischemic stroke, acute coronary syndrome, or acute limb ischemia. N-Acetylcysteine (NAC, a clinically approved mucolytic drug) can reduce intrachain disulfide bonds in large polymeric proteins. In the present study, we postulated that NAC might cleave the VWF multimers inside occlusive thrombi, thereby leading to their dissolution and arterial recanalization.
METHODS: Experimental models of thrombotic stroke induced by either intra-arterial thrombin injection or ferric chloride application followed by measurement of cerebral blood flow using a combination of laser Doppler flowmetry and MRI were performed to uncover the effects of NAC on arterial thrombi. To investigate the effect of NAC on larger vessels, we also performed ferric chloride-induced carotid artery thrombosis. In vitro experiments were performed to study the molecular bases of NAC thrombolytic effect, including platelet aggregometry, platelet-rich thrombi lysis assays, thromboelastography (ROTEM), and high-shear VWF string formation using microfluidic devices. We also investigated the putative prohemorrhagic effect of NAC in a mouse model of intracranial hemorrhage induced by in situ collagenase type VII injection.
RESULTS: We demonstrated that intravenous NAC administration promotes lysis of arterial thrombi that are resistant to conventional approaches such as recombinant tissue-type plasminogen activator, direct thrombin inhibitors, and antiplatelet treatments. Through in vitro and in vivo experiments, we provide evidence that the molecular target underlying the thrombolytic effects of NAC is principally the VWF that cross-link platelets in arterial thrombi. Coadministration of NAC and a nonpeptidic GpIIb/IIIa inhibitor further improved its thrombolytic efficacy, essentially by accelerating thrombus dissolution and preventing rethrombosis. Thus, in a new large-vessel thromboembolic stroke model in mice, this cotreatment significantly improved ischemic lesion size and neurological outcome. It is important to note that NAC did not worsen hemorrhagic stroke outcome, suggesting that it exerts thrombolytic effects without significantly impairing normal hemostasis.
CONCLUSIONS: We provide evidence that NAC is an effective and safe alternative to currently available antithrombotic agents to restore vessel patency after arterial occlusion.
References
Adam, Casari, Prévost, Kauskot, Loubière et al., A genetically-engineered von Willebrand disease type 2B mouse model displays defects in hemostasis and inflammation, Sci Rep, doi:10.1038/srep26306
Alexander, None, European Society of Cardiology Congress
Arranz, Fernández, Rodríguez, Ribera, De La Fuente, The glutathione precursor N-acetylcysteine improves immune function in postmenopausal women, Free Radic Biol Med, doi:10.1016/j.freeradbiomed.2008.07.014
Arstall, Yang, Stafford, Betts, Horowitz, N-Acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects, Circulation
Behot, Gauberti, De Lizarrondo, Montagne, Lemarchand et al., GpIbα-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice, Blood, doi:10.1182/blood-2013-12-543074
Bhatia, Hill, Shobha, Menon, Bal et al., Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action, Stroke, doi:10.1161/STROKEAHA.110.592535
Blankenberg, Rupprecht, Bickel, Torzewski, Hafner et al., Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease, N Engl J Med, doi:10.1056/NEJMoa030535
Cabanillas, Popescu-Martinez, N-Acetylcysteine for relapsing thrombotic thrombocytopenic purpura: more evidence of a promising drug, Am J Ther, doi:10.1097/MJT.0000000000000386
Chen, Reheman, Gushiken, Nolasco, Fu et al., N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice, J Clin Invest, doi:10.1172/JCI41062
Colace, Diamond, Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.112.300522
Crescente, Thomas, Demers, Voorhees, Wong et al., ADAMTS13 exerts a thrombolytic effect in microcirculation, Thromb Haemost, doi:10.1160/TH12-01-0046
Denis, Methia, Frenette, Rayburn, Ullman-Culleré et al., A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis, Proc Natl Acad Sci
Denorme, Langhauser, Desender, Vandenbulcke, Rottensteiner et al., ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice, Blood, doi:10.1182/blood-2015-08-662650
Dirnagl, Klehmet, Braun, Harms, Meisel et al., Stroke-induced immunodepression: experimental evidence and clinical relevance, Stroke, doi:10.1161/01.STR.0000251441.89665.bc
Gaberel, Gakuba, Hebert, Montagne, Agin et al., Intracerebral hematomas disappear on T2*-weighted images during normobaric oxygen therapy, Stroke, doi:10.1161/STROKEAHA.113.002045
Gakuba, Gauberti, Mazighi, Defer, Hanouz et al., Preclinical evidence toward the use of ketamine for recombinant tissue-type plasminogen activator-mediated thrombolysis under anesthesia or sedation, Stroke, doi:10.1161/STROKEAHA.111.620468
Gauberti, Montagne, Contreras, Béhot, Maubert et al., Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes, Stroke, doi:10.1161/STROKEAHA.111.000544
George, López, Konkle, N-Acetylcysteine: an old drug, a new insight, a potentially effective treatment for thrombotic thrombocytopenic purpura, Transfusion, doi:10.1111/trf.12561
Herbig, Diamond, Pathological von Willebrand factor fibers resist tissue plasminogen activator and ADAMTS13 while promoting the contact pathway and shear-induced platelet activation, J Thromb Haemost, doi:10.1111/jth.13044
Hussain, Varelogianni, Särndahl, Roomans, N-acetylcysteine and azithromycin affect the innate immune response in cystic fibrosis bronchial epithelial cells in vitro, Exp Lung Res, doi:10.3109/01902148.2014.934411
Karatas, Erdener, Gursoy-Ozdemir, Gurer, Soylemezoglu et al., Thrombotic distal middle cerebral artery occlusion produced by topical FeCl(3) application: a novel model suitable for intravital microscopy and thrombolysis studies, J Cereb Blood Flow Metab, doi:10.1038/jcbfm.2011.8
Kerr, Dawson, Whyte, Buckley, Murray et al., The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine, Ann Emerg Med, doi:10.1016/j.annemergmed.2004.08.040
Kim, Heo, Lee, Kim, Suh et al., Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT, Neurology, doi:10.1212/01.wnl.0000244492.99737.a8
Li, Lin, Zhang, Wu, Liu et al., Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients, Stroke, doi:10.1161/STROKEAHA.116.014413
Nesbitt, Westein, Tovar-Lopez, Tolouei, Mitchell et al., A shear gradient-dependent platelet aggregation mechanism drives thrombus formation, Nat Med, doi:10.1038/nm.1955
Niemi, Munsterhjelm, Pöyhiä, Hynninen, Salmenperä, The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm, Blood Coagul Fibrinolysis, doi:10.1097/01.mbc.0000195922.26950.89
Oa, De Lizarrondo, Bardou, Orset, Pruvost et al., Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin-and bradykinin-dependent mechanism, Blood, doi:10.1182/blood-2016-03-705384
Orset, Macrez, Young, Panthou, Angles-Cano et al., Mouse model of in situ thromboembolic stroke and reperfusion, Stroke, doi:10.1161/STROKEAHA.107.487520
Ruggeri, Orje, Habermann, Federici, Reininger, Activation-independent platelet adhesion and aggregation under elevated shear stress, Blood, doi:10.1182/blood-2006-04-011551
Shahripour, Harrigan, Alexandrov, N-Acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities, Brain Behav, doi:10.1002/brb3.208
Siebler, Hennerici, Schneider, Von Reutern, Seitz et al., Safety of tirofiban in acute ischemic stroke: the SaTIS trial, Stroke, doi:10.1161/STROKEAHA.110.599662
Vivien, Gauberti, Montagne, Defer, Touzé, Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence, J Cereb Blood Flow Metab, doi:10.1038/jcbfm.2011.127
Víctor, Rocha, De La Fuente, Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin, Int Immunopharmacol
DOI record:
{
"DOI": "10.1161/circulationaha.117.027290",
"ISSN": [
"0009-7322",
"1524-4539"
],
"URL": "http://dx.doi.org/10.1161/CIRCULATIONAHA.117.027290",
"abstract": "<jats:sec>\n <jats:title>Background:</jats:title>\n <jats:p>\n Platelet cross-linking during arterial thrombosis involves von Willebrand Factor (VWF) multimers. Therefore, proteolysis of VWF appears promising to disaggregate platelet-rich thrombi and restore vessel patency in acute thrombotic disorders such as ischemic stroke, acute coronary syndrome, or acute limb ischemia.\n <jats:italic>N</jats:italic>\n -Acetylcysteine (NAC, a clinically approved mucolytic drug) can reduce intrachain disulfide bonds in large polymeric proteins. In the present study, we postulated that NAC might cleave the VWF multimers inside occlusive thrombi, thereby leading to their dissolution and arterial recanalization.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods:</jats:title>\n <jats:p>Experimental models of thrombotic stroke induced by either intra-arterial thrombin injection or ferric chloride application followed by measurement of cerebral blood flow using a combination of laser Doppler flowmetry and MRI were performed to uncover the effects of NAC on arterial thrombi. To investigate the effect of NAC on larger vessels, we also performed ferric chloride–induced carotid artery thrombosis. In vitro experiments were performed to study the molecular bases of NAC thrombolytic effect, including platelet aggregometry, platelet-rich thrombi lysis assays, thromboelastography (ROTEM), and high-shear VWF string formation using microfluidic devices. We also investigated the putative prohemorrhagic effect of NAC in a mouse model of intracranial hemorrhage induced by in situ collagenase type VII injection.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results:</jats:title>\n <jats:p>We demonstrated that intravenous NAC administration promotes lysis of arterial thrombi that are resistant to conventional approaches such as recombinant tissue-type plasminogen activator, direct thrombin inhibitors, and antiplatelet treatments. Through in vitro and in vivo experiments, we provide evidence that the molecular target underlying the thrombolytic effects of NAC is principally the VWF that cross-link platelets in arterial thrombi. Coadministration of NAC and a nonpeptidic GpIIb/IIIa inhibitor further improved its thrombolytic efficacy, essentially by accelerating thrombus dissolution and preventing rethrombosis. Thus, in a new large-vessel thromboembolic stroke model in mice, this cotreatment significantly improved ischemic lesion size and neurological outcome. It is important to note that NAC did not worsen hemorrhagic stroke outcome, suggesting that it exerts thrombolytic effects without significantly impairing normal hemostasis.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions:</jats:title>\n <jats:p>We provide evidence that NAC is an effective and safe alternative to currently available antithrombotic agents to restore vessel patency after arterial occlusion.</jats:p>\n </jats:sec>",
"alternative-id": [
"10.1161/CIRCULATIONAHA.117.027290"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2016-03-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2017-04-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2017-05-09"
}
],
"author": [
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Martinez de Lizarrondo",
"given": "Sara",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Gakuba",
"given": "Clément",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Herbig",
"given": "Bradley A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Repessé",
"given": "Yohann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Ali",
"given": "Carine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Denis",
"given": "Cécile V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Lenting",
"given": "Peter J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Touzé",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Diamond",
"given": "Scott L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Vivien",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Normandie Univ, UNICAEN, INSERM, INSERM UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Cyceron, Caen, France (S.M.d.L., C.G., Y.R., C.A., E.T., D.V., M.G.); CHU de Caen, Department of Anesthesiology and Critical Care Medicine, CHU de Caen Côte de Nacre, France (C.G.); Institute for Medicine and Engineering, Department of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia (B.A.H., S.L.D.); Laboratoire d’Hématologie, CHU de Caen, France (Y..."
}
],
"family": "Gauberti",
"given": "Maxime",
"sequence": "additional"
}
],
"container-title": "Circulation",
"container-title-short": "Circulation",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.ahajournals.org"
]
},
"created": {
"date-parts": [
[
2017,
5,
10
]
],
"date-time": "2017-05-10T00:30:29Z",
"timestamp": 1494376229000
},
"deposited": {
"date-parts": [
[
2024,
5,
13
]
],
"date-time": "2024-05-13T06:54:41Z",
"timestamp": 1715583281000
},
"indexed": {
"date-parts": [
[
2025,
9,
28
]
],
"date-time": "2025-09-28T15:24:56Z",
"timestamp": 1759073096045
},
"is-referenced-by-count": 130,
"issue": "7",
"issued": {
"date-parts": [
[
2017,
8,
15
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2017,
8,
15
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.117.027290",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "646-660",
"prefix": "10.1161",
"published": {
"date-parts": [
[
2017,
8,
15
]
]
},
"published-print": {
"date-parts": [
[
2017,
8,
15
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1038/jcbfm.2011.127",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_2_2"
},
{
"DOI": "10.1161/STROKEAHA.110.592535",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.1212/01.wnl.0000244492.99737.a8",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1182/blood-2013-12-543074",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1038/nm.1955",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1182/blood-2006-04-011551",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.1182/blood-2015-08-662650",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.1160/TH12-01-0046",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_9_2"
},
{
"DOI": "10.1172/JCI41062",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_2"
},
{
"DOI": "10.1073/pnas.95.16.9524",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_2"
},
{
"DOI": "10.1038/jcbfm.2011.8",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"DOI": "10.1161/STROKEAHA.107.487520",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_2"
},
{
"DOI": "10.1161/STROKEAHA.111.000544",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_2"
},
{
"DOI": "10.1161/STROKEAHA.113.002045",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"DOI": "10.1161/STROKEAHA.111.620468",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_2"
},
{
"DOI": "10.1111/jth.13044",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1161/ATVBAHA.112.300522",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1182/blood-2016-03-705384",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1038/srep26306",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.1161/STROKEAHA.116.014413",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1097/MJT.0000000000000386",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"DOI": "10.1111/trf.12561",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_2"
},
{
"DOI": "10.1161/01.CIR.92.10.2855",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_24_2"
},
{
"article-title": "European Society of Cardiology Congress.",
"author": "Alexander W",
"first-page": "645",
"journal-title": "P T",
"key": "e_1_3_4_25_2",
"unstructured": "Alexander W. European Society of Cardiology Congress. P T. 2016;41:645–649.",
"volume": "41",
"year": "2016"
},
{
"DOI": "10.1002/brb3.208",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_26_2"
},
{
"DOI": "10.1097/01.mbc.0000195922.26950.89",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_27_2"
},
{
"DOI": "10.1056/NEJMoa030535",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_28_2"
},
{
"DOI": "10.1161/STROKEAHA.110.599662",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_29_2"
},
{
"DOI": "10.1016/j.freeradbiomed.2008.07.014",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_30_2"
},
{
"DOI": "10.1016/S1567-5769(02)00232-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_31_2"
},
{
"DOI": "10.3109/01902148.2014.934411",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_32_2"
},
{
"DOI": "10.1016/j.annemergmed.2004.08.040",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_33_2"
},
{
"DOI": "10.1161/01.STR.0000251441.89665.bc",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_34_2"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.117.027290"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Potent Thrombolytic Effect of\n <i>N</i>\n -Acetylcysteine on Arterial Thrombi",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1161/crossmarkpolicy",
"volume": "136"
}
