Safety and Outcomes Associated with the Pharmacological Inhibition of the Kinin–Kallikrein System in Severe COVID-19
et al., Viruses, doi:10.3390/v13020309, Feb 2021
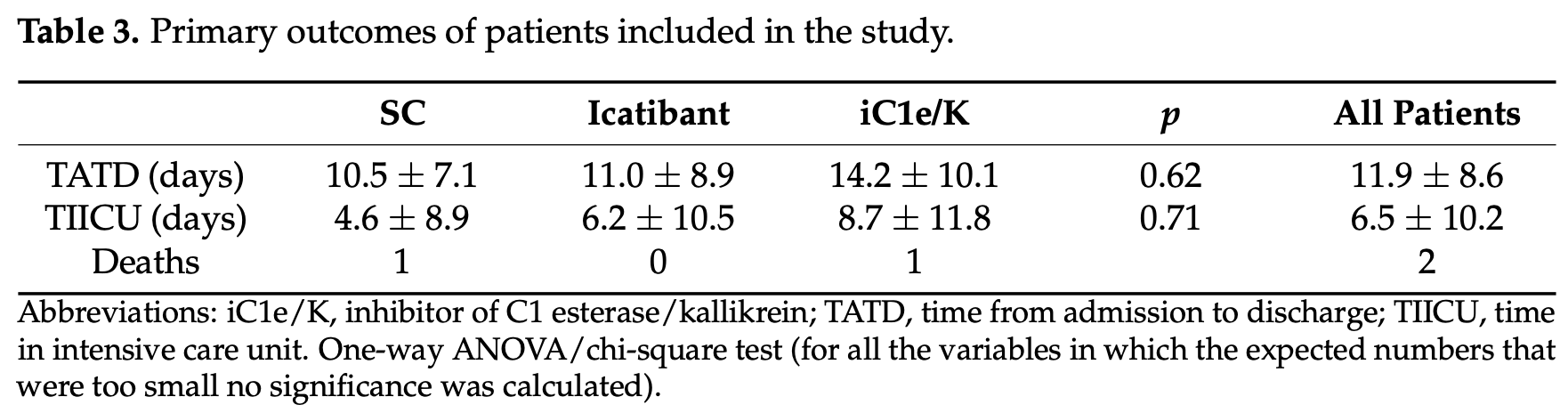
RCT 30 severe COVID-19 patients showing no significant difference in mortality or hospitalization time with kinin-kallikrein system inhibitors icatibant or iC1e/K. While there was no impact on mortality or hospitalization time, both treatments were safe and showed improvements in lung CT scores and increased blood eosinophil counts.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 1 of 10 (10.0%), control 1 of 10 (10.0%).
|
|
time to discharge, 35.2% higher, relative time 1.35, p = 0.36, treatment mean 14.2 (±10.1) n=10, control mean 10.5 (±7.1) n=10.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mansour et al., 16 Feb 2021, Randomized Controlled Trial, Brazil, peer-reviewed, mean age 51.6, 20 authors, study period 22 April, 2020 - 14 June, 2020.
Contact: lavellos@unicamp.br (corresponding author), eliemansour27@hotmail.com, palma.andrecitroni@gmail.com, haysa_@hotmail.com, costaribeirolu@gmail.com, anaflavia.bsousa@gmail.com, thya_goo@hotmail.com, marcus.agrela@gmail.com, santosth@unicamp.br, trabasso@gmail.com, lmoretti@unicamp.br, brunabombassaro@gmail.com, monfortpiresm@gmail.com, rlcamargo.bio@gmail.com, pa.eliana@gmail.com, nbrunetti.bio@gmail.com, asfarias@unicamp.br, aefalcao@gmail.com, rakapolo@yahoo.com, dertkigil@uol.com.br.
Safety and Outcomes Associated with the Pharmacological Inhibition of the Kinin–Kallikrein System in Severe COVID-19
Viruses, doi:10.3390/v13020309
Background: Coronavirus disease 19 (COVID-19) can develop into a severe respiratory syndrome that results in up to 40% mortality. Acute lung inflammatory edema is a major pathological finding in autopsies explaining O 2 diffusion failure and hypoxemia. Only dexamethasone has been shown to reduce mortality in severe cases, further supporting a role for inflammation in disease severity. SARS-CoV-2 enters cells employing angiotensin-converting enzyme 2 (ACE2) as a receptor, which is highly expressed in lung alveolar cells. ACE2 is one of the components of the cellular machinery that inactivates the potent inflammatory agent bradykinin, and SARS-CoV-2 infection could interfere with the catalytic activity of ACE2, leading to the accumulation of bradykinin. Methods: In this case control study, we tested two pharmacological inhibitors of the kinin-kallikrein system that are currently approved for the treatment of hereditary angioedema, icatibant, and inhibitor of C1 esterase/kallikrein, in a group of 30 patients with severe COVID-19. Results: Neither icatibant nor inhibitor of C1 esterase/kallikrein resulted in changes in time to clinical improvement. However, both compounds were safe and promoted the significant improvement of lung computed tomography scores and increased blood eosinophils, which are indicators of disease recovery. Conclusions: In this small cohort, we found evidence for safety and a beneficial role of pharmacological inhibition of the kinin-kallikrein system in two markers that indicate improved disease recovery.
Supplementary Materials: The following are available at https://www.mdpi.com/1999-4915/13 /2/309/s1 , Table S1 : Patients screened and excluded from study; Table S2 : Patients included in the study; Table S3 : Supporting medication; Table S4 : Blood cell counts; Table S5 : Blood coagulation parameter values; Table S6 : Parameters related to renal function; Table S7 : Adverse effects during intervention; Table S8 : Clinical variables at discharge; Supplementary Figure S1 : Computed tomography lung scans obtained from patients randomized to standard care; Supplementary Figure S2 : Computed tomography lung scans obtained from patients randomized to icatibant; Supplementary Figure S3 : Computed tomography lung scans obtained from patients randomized to inhibitor of C1 esterase/kallikrein (iC1e/K). Author Contributions: L.A.V. is the supervisor and lead contact. L.A.V. conceived the study, raised funds, organized the protocol, and wrote the paper. E.M. coordinated the execution team and provided thoughtful insights for the design of the study. M.L.M. was the co-supervisor of the study. Institutional Review Board Statement: Ethical Review Committee of the Clinics Hospital of the University of Campinas (protocol CAEE: 30227920.9.0000.5404).
Informed Consent Statement: Written consent to participate in this study has been obtained from all patients or substitute decisionmakers (for patients lacking decision-making capacity).
Conflicts of
References
Acharya, Liu, Gack, Dysregulation of type I interferon responses in COVID-19, Nat. Rev. Immunol, doi:10.1038/s41577-020-0346-x
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2015432
Barton, Duval, Stroberg, Ghosh, Mukhopadhyay et al., None, Am. J. Clin. Pathol, doi:10.1093/ajcp/aqaa062
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Boccon-Gibod, Bouillet, Safety and efficacy of icatibant self-administration for acute hereditary angioedema, Clin. Exp. Immunol, doi:10.1111/j.1365-2249.2012.04574.x
Boehm, Nabel, Angiotensin-converting enzyme 2-A new cardiac regulator, N. Engl. J. Med, doi:10.1056/NEJMcibr022472
Borba, Val, Sampaio, Alexandre, Melo et al., Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.8857
Bork, Frank, Grundt, Schlattmann, Nussberger et al., Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant), J Allergy Clin. Immunol, doi:10.1016/j.jaci.2007.02.012
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2001282
Chen, Xiong, Bao, Shi, Convalescent plasma as a potential therapy for COVID-19, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30141-9
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Chusid, Eosinophils: Friends or Foes?, J. Allergy Clin. Immunol. Pract, doi:10.1016/j.jaip.2018.04.031
Cocchio, Marzella, Cinryze, a human plasma-derived c1 esterase inhibitor for prophylaxis of hereditary angioedema, Pharm. Ther
Cohen, Corey, Combination prevention for COVID-19, Science, doi:10.1126/science.abc5798
Connors, Levy, COVID-19 and its implications for thrombosis and anticoagulation, Blood, doi:10.1182/blood.2020006000
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Eur. Surveill, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Du, Tu, Zhu, Mu, Wang et al., Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.202003-0543OC
Fine, Fogarty, Linz, Letter: Fresh frozen plasma for prophylaxis in hereditary angioedema, J. Allergy Clin. Immunol, doi:10.1016/0091-6749(76)90017-8
Fischer, Lamer, Wang, Mckinnie, Iturrioz et al., Plasma kallikrein cleaves and inactivates apelin-17: Palmitoyl-and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents, Eur. J. Med. Chem
Franco, Rivas-Santisteban, Serrano-Marin, Rodriguez-Perez, Labandeira-Garcia et al., SARS-CoV-2 as a Factor to Disbalance the Renin-Angiotensin System: A Suspect in the Case of Exacerbated IL-6 Production, J. Immunol, doi:10.4049/jimmunol.2000642
Fu, Lou, Xi, Bai, Ma et al., Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia, Eur. Radiol, doi:10.1007/s00330-020-06920-8
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19-Preliminary Report, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Guy, Dipaola, Romanelli, Dutch, Rapid repurposing of drugs for COVID-19, Science, doi:10.1126/science.abb9332
Hadjadj, Yatim, Barnabei, Corneau, Boussier et al., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science, doi:10.1126/science.abc6027
Hung, Lung, Tso, Liu, Chung et al., Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial, Lancet, doi:10.1016/S0140-6736(20)31042-4
Jiang, Don't rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees, Nature, doi:10.1038/d41586-020-00751-9
Kuba, Imai, Rao, Gao, Guo et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat. Med, doi:10.1038/nm1267
Leach, Spencer, Mascelli, Mccauley, Pharmacokinetics of single and repeat doses of icatibant, Clin. Pharmacol. Drug Dev, doi:10.1002/cpdd.138
Lee, Jacobsen, Mcgarry, Schleimer, Lee, Eosinophils in health and disease: The LIAR hypothesis, Clin. Exp. Allergy, doi:10.1111/j.1365-2222.2010.03484.x
Lumry, Li, Levy, Potter, Farkas et al., Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: The FAST-3 trial, Ann. Allergy Asthma. Immunol, doi:10.1016/j.anai.2011.08.015
Martinez De Lizarrondo, Bardou, Orset, Pruvost, Anfray et al., Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin-and bradykinindependent mechanism, Blood, doi:10.1182/blood-2016-03-705384
Martinez-Saguer, Cicardi, Suffritti, Rusicke, Aygoren-Pursun et al., Pharmacokinetics of plasma-derived C1-esterase inhibitor after subcutaneous versus intravenous administration in subjects with mild or moderate hereditary angioedema: The PASSION study, Transfusion, doi:10.1111/trf.12501
Mendes, Jara, Mansour, Araujo, Velloso, Asthma and COVID-19: A systematic review, Allergy Asthma Clin. Immunol, doi:10.1186/s13223-020-00509-y
Mogielnicki, Kramkowski, Hermanowicz, Leszczynska, Przyborowski et al., Angiotensin-(1-9) enhances stasis-induced venous thrombosis in the rat because of the impairment of fibrinolysis, J. Renin. Angiotensin. Aldosterone Syst, doi:10.1177/1470320313498631
Monteil, Kwon, Prado, Hagelkruys, Wimmer et al., Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2, Cell, doi:10.1016/j.cell.2020.04.004
Nussberger, Cugno, Amstutz, Cicardi, Pellacani et al., Plasma bradykinin in angio-oedema, Lancet, doi:10.1016/S0140-6736(97)09137-X
Nussberger, Cugno, Cicardi, Bradykinin-mediated angioedema, N. Engl. J. Med, doi:10.1056/NEJM200208223470820
Pan, Ye, Sun, Gui, Liang et al., Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19), Radiology, doi:10.1148/radiol.2020200370
Petho, Reeh, Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors, Physiol. Rev, doi:10.1152/physrev.00048.2010
Pickering, Good, Kelly, Gewurz, Replacement therapy in hereditary angioedema. Successful treatment of two patients with fresh frozen plasma, Lancet, doi:10.1016/S0140-6736(69)91295-1
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Roberts, Pandemic brings mass vaccinations to a halt, Science, doi:10.1126/science.368.6487.116
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet, doi:10.1016/S0140-6736(20)30317-2
Salehi, Abedi, Balakrishnan, Gholamrezanezhad, Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: A proposal based on the imaging data of 37 studies, Eur. Radiol, doi:10.1007/s00330-020-06863-0
Samarasinghe, Melo, Duan, Lemessurier, Liedmann et al., Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus, J. Immunol, doi:10.4049/jimmunol.1600787
Sarzani, Giulietti, Di Pentima, Giordano, Spannella, Disequilibrium between the Classic Renin-Angiotensin System and Its Opposing Arm in Sars-Cov-2 Related Lung Injury, Am. J. Physiol. Lung Cell Mol. Physiol, doi:10.1152/ajplung.00189.2020
Schmieder, Hilgers, Schlaich, Schmidt, Renin-angiotensin system and cardiovascular risk, Lancet, doi:10.1016/S0140-6736(07)60242-6
Shang, Wan, Luo, Ye, Geng et al., Cell entry mechanisms of SARS-CoV-2, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2003138117
Shi, Shan, Duan, Chen, Liu et al., A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2, Nature, doi:10.1038/s41586-020-2381-y
Sidarta-Oliveira, Jara, Ferruzzi, Skaf, Velander et al., SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cells, Sci. Rep, doi:10.1038/s41598-020-76488-2
Sodhi, Wohlford-Lenane, Yamaguchi, Prindle, Fulton et al., Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration, Am. J. Physiol. Lung Cell Mol. Physiol, doi:10.1152/ajplung.00498.2016
Song, Dai, Jang, Medrano, Li et al., Low-and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue, J. Clin. Investig, doi:10.1172/JCI129167
Sturrock, Milne, Chevassut, The renin-angiotensin system-A therapeutic target in COVID-19?, Clin. Med, doi:10.7861/clinmed.2020-0146
Van De Veerdonk, Kouijzer, De Nooijer, Van Der Hoeven, Maas et al., Outcomes Associated With Use of a Kinin B2 Receptor Antagonist Among Patients With COVID-19, JAMA Net. Open, doi:10.1001/jamanetworkopen.2020.17708
Van De Veerdonk, Netea, Van Deuren, Van Der Meer, De Mast et al., Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome, Elife, doi:10.7554/eLife.57555
Velloso, Folli, Sun, White, Saad et al., Cross-talk between the insulin and angiotensin signaling systems, Proc. Natl. Acad. Sci, doi:10.1073/pnas.93.22.12490
Wang, Fan, Salam, Horby, Hayden et al., Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection, J. Infect. Dis, doi:10.1093/infdis/jiz656
Wang, Saguner, An, Ning, Yan et al., Dysfunctional Coagulation in COVID-19: From Cell to Bedside, Adv. Ther, doi:10.1007/s12325-020-01399-7
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Yan, Zhang, Li, Xia, Guo et al., Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2, Science, doi:10.1126/science.abb2762
Zhao, Zhang, Yang, Liu, Eosinopenia is associated with greater severity in patients with coronavirus disease 2019, Allergy, doi:10.1111/all.14455
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zhou, Zhu, Wang, Xia, Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China, Eur. Radiol, doi:10.1007/s00330-020-06879-6
Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
Ziegler, Allon, Nyquist, Mbano, Miao et al., SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues, Cell, doi:10.1016/j.cell.2020.04.035
Zuraw, Cicardi, Longhurst, Bernstein, Li et al., Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate, Allergy, doi:10.1111/all.12658
DOI record:
{
"DOI": "10.3390/v13020309",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v13020309",
"abstract": "<jats:p>Background: Coronavirus disease 19 (COVID-19) can develop into a severe respiratory syndrome that results in up to 40% mortality. Acute lung inflammatory edema is a major pathological finding in autopsies explaining O2 diffusion failure and hypoxemia. Only dexamethasone has been shown to reduce mortality in severe cases, further supporting a role for inflammation in disease severity. SARS-CoV-2 enters cells employing angiotensin-converting enzyme 2 (ACE2) as a receptor, which is highly expressed in lung alveolar cells. ACE2 is one of the components of the cellular machinery that inactivates the potent inflammatory agent bradykinin, and SARS-CoV-2 infection could interfere with the catalytic activity of ACE2, leading to the accumulation of bradykinin. Methods: In this case control study, we tested two pharmacological inhibitors of the kinin–kallikrein system that are currently approved for the treatment of hereditary angioedema, icatibant, and inhibitor of C1 esterase/kallikrein, in a group of 30 patients with severe COVID-19. Results: Neither icatibant nor inhibitor of C1 esterase/kallikrein resulted in changes in time to clinical improvement. However, both compounds were safe and promoted the significant improvement of lung computed tomography scores and increased blood eosinophils, which are indicators of disease recovery. Conclusions: In this small cohort, we found evidence for safety and a beneficial role of pharmacological inhibition of the kinin–kallikrein system in two markers that indicate improved disease recovery.</jats:p>",
"alternative-id": [
"v13020309"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-6450-6930",
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Mansour",
"given": "Eli",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-6823-0847",
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Palma",
"given": "Andre C.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0013-2831",
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Ulaf",
"given": "Raisa G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Ribeiro",
"given": "Luciana C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Bernardes",
"given": "Ana Flavia",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0202-0550",
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Nunes",
"given": "Thyago A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Agrela",
"given": "Marcus V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Obesity and Comorbidities Research Center, University of Campinas, 13083-864 Campinas, São Paulo, Brazil"
}
],
"family": "Bombassaro",
"given": "Bruna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Obesity and Comorbidities Research Center, University of Campinas, 13083-864 Campinas, São Paulo, Brazil"
}
],
"family": "Monfort-Pires",
"given": "Milena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Obesity and Comorbidities Research Center, University of Campinas, 13083-864 Campinas, São Paulo, Brazil"
}
],
"family": "Camargo",
"given": "Rafael L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Obesity and Comorbidities Research Center, University of Campinas, 13083-864 Campinas, São Paulo, Brazil"
},
{
"name": "School of Nursing, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Araujo",
"given": "Eliana P.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2803-3763",
"affiliation": [
{
"name": "Autoimmune Research Lab, Department of Genetics, Microbiology and Immunology, Institute of Biology, University of Campinas, 13083-862 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Brunetti",
"given": "Natalia S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Autoimmune Research Lab, Department of Genetics, Microbiology and Immunology, Institute of Biology, University of Campinas, 13083-862 Campinas, São Paulo, Brazil"
}
],
"family": "Farias",
"given": "Alessandro S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Falcão",
"given": "Antônio Luís E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Santos",
"given": "Thiago Martins",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Trabasso",
"given": "Plinio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"family": "Dertkigil",
"given": "Rachel P.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3694-9539",
"affiliation": [
{
"name": "Department of Radiology, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Dertkigil",
"given": "Sergio S.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2280-5649",
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
}
],
"authenticated-orcid": false,
"family": "Moretti",
"given": "Maria Luiza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medical Sciences, University of Campinas, 13083-887 Campinas, São Paulo, Brazil"
},
{
"name": "Obesity and Comorbidities Research Center, University of Campinas, 13083-864 Campinas, São Paulo, Brazil"
}
],
"family": "Velloso",
"given": "Licio A.",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
16
]
],
"date-time": "2021-02-16T13:09:09Z",
"timestamp": 1613480949000
},
"deposited": {
"date-parts": [
[
2024,
7,
8
]
],
"date-time": "2024-07-08T08:01:11Z",
"timestamp": 1720425671000
},
"funder": [
{
"DOI": "10.13039/501100001807",
"award": [
"2013/07607"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001807",
"id-type": "DOI"
}
],
"name": "Fundação de Amparo à Pesquisa do Estado de São Paulo"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
9
]
],
"date-time": "2025-04-09T13:52:37Z",
"timestamp": 1744206757842,
"version": "3.37.3"
},
"is-referenced-by-count": 39,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
16
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2021,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
16
]
],
"date-time": "2021-02-16T00:00:00Z",
"timestamp": 1613433600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/13/2/309/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "309",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
2,
16
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
16
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "JAMA",
"key": "ref_1",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "ref_2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Lancet",
"key": "ref_3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s00330-020-06920-8",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Fu, F., Lou, J., Xi, D., Bai, Y., Ma, G., Zhao, B., Liu, D., Bao, G., Lei, Z., and Wang, M. (2020). Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. Eur. Radiol."
},
{
"DOI": "10.1007/s00330-020-06863-0",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Salehi, S., Abedi, A., Balakrishnan, S., and Gholamrezanezhad, A. (2020). Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: A proposal based on the imaging data of 37 studies. Eur. Radiol."
},
{
"DOI": "10.1056/NEJMoa2015432",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Ackermann, M., Verleden, S.E., Kuehnel, M., Haverich, A., Welte, T., Laenger, F., Vanstapel, A., Werlein, C., Stark, H., and Tzankov, A. (2020). Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med."
},
{
"DOI": "10.1038/s41577-020-0346-x",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Acharya, D., Liu, G., and Gack, M.U. (2020). Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol."
},
{
"key": "ref_8",
"unstructured": "Group, R.C., Horby, P., Lim, W.S., Emberson, J.R., Mafham, M., Bell, J.L., Linsell, L., Staplin, N., Brightling, C., and Ustianowski, A. (2020). Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med."
},
{
"DOI": "10.1016/S0140-6736(20)30317-2",
"article-title": "Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Lancet",
"key": "ref_9",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"article-title": "Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial",
"author": "Borba",
"doi-asserted-by": "crossref",
"first-page": "e208857",
"journal-title": "JAMA Netw. Open",
"key": "ref_10",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N. Engl. J. Med.",
"key": "ref_11",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "ref_12",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1172/JCI129167",
"article-title": "Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "247",
"journal-title": "J. Clin. Investig.",
"key": "ref_13",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2762",
"article-title": "Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "1444",
"journal-title": "Science",
"key": "ref_14",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.035",
"article-title": "SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues",
"author": "Ziegler",
"doi-asserted-by": "crossref",
"first-page": "1016",
"journal-title": "Cell",
"key": "ref_15",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-76488-2",
"article-title": "SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cells",
"author": "Jara",
"doi-asserted-by": "crossref",
"first-page": "19522",
"journal-title": "Sci. Rep.",
"key": "ref_16",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"article-title": "Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1036",
"journal-title": "Cell",
"key": "ref_17",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1101/2020.04.19.20068015",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Hadjadj, J., Yatim, N., Barnabei, L., Corneau, A., Boussier, J., Smith, N., Pere, H., Charbit, B., Bondet, V., and Chenevier-Gobeaux, C. (2020). Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science."
},
{
"DOI": "10.1126/science.abc5798",
"article-title": "Combination prevention for COVID-19",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "551",
"journal-title": "Science",
"key": "ref_19",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1038/d41586-020-00751-9",
"article-title": "Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "321",
"journal-title": "Nature",
"key": "ref_20",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1126/science.368.6487.116",
"article-title": "Pandemic brings mass vaccinations to a halt",
"author": "Roberts",
"doi-asserted-by": "crossref",
"first-page": "116",
"journal-title": "Science",
"key": "ref_21",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "ref_22",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1126/science.abb9332",
"article-title": "Rapid repurposing of drugs for COVID-19",
"author": "Guy",
"doi-asserted-by": "crossref",
"first-page": "829",
"journal-title": "Science",
"key": "ref_23",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"article-title": "Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "1695",
"journal-title": "Lancet",
"key": "ref_24",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2381-y",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Shi, R., Shan, C., Duan, X., Chen, Z., Liu, P., Song, J., Song, T., Bi, X., Han, C., and Wu, L. (2020). A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature."
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"article-title": "Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2",
"author": "Monteil",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Cell",
"key": "ref_26",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.7554/eLife.57555",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "van de Veerdonk, F.L., Netea, M.G., van Deuren, M., van der Meer, J.W., de Mast, Q., Bruggemann, R.J., and van der Hoeven, H. (2020). Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife, 9."
},
{
"DOI": "10.4049/jimmunol.2000642",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Franco, R., Rivas-Santisteban, R., Serrano-Marin, J., Rodriguez-Perez, A.I., Labandeira-Garcia, J.L., and Navarro, G. (2020). SARS-CoV-2 as a Factor to Disbalance the Renin-Angiotensin System: A Suspect in the Case of Exacerbated IL-6 Production. J. Immunol."
},
{
"DOI": "10.1152/ajplung.00189.2020",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Sarzani, R., Giulietti, F., Di Pentima, C., Giordano, P., and Spannella, F. (2020). Disequilibrium between the Classic Renin-Angiotensin System and Its Opposing Arm in Sars-Cov-2 Related Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol."
},
{
"DOI": "10.1007/s12325-020-01399-7",
"article-title": "Dysfunctional Coagulation in COVID-19: From Cell to Bedside",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "3033",
"journal-title": "Adv. Ther.",
"key": "ref_30",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00498.2016",
"article-title": "Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration",
"author": "Sodhi",
"doi-asserted-by": "crossref",
"first-page": "L17",
"journal-title": "Am. J. Physiol. Lung Cell Mol. Physiol.",
"key": "ref_31",
"volume": "314",
"year": "2018"
},
{
"DOI": "10.1073/pnas.2003138117",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., and Li, F. (2020). Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA."
},
{
"DOI": "10.7861/clinmed.2020-0146",
"doi-asserted-by": "crossref",
"key": "ref_33",
"unstructured": "Sturrock, B.R., Milne, K., and Chevassut, T.J. (2020). The renin-angiotensin system—A therapeutic target in COVID-19?. Clin. Med."
},
{
"DOI": "10.1182/blood.2020006000",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Connors, J.M., and Levy, J.H. (2020). COVID-19 and its implications for thrombosis and anticoagulation. Blood."
},
{
"DOI": "10.1038/nm1267",
"article-title": "A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury",
"author": "Kuba",
"doi-asserted-by": "crossref",
"first-page": "875",
"journal-title": "Nat. Med.",
"key": "ref_35",
"volume": "11",
"year": "2005"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Corman, V.M., Landt, O., Kaiser, M., Molenkamp, R., Meijer, A., Chu, D.K., Bleicker, T., Brunink, S., Schneider, J., and Schmidt, M.L. (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eur. Surveill, 25."
},
{
"DOI": "10.1148/radiol.2020200370",
"article-title": "Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19)",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Radiology",
"key": "ref_37",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_38",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiz656",
"article-title": "Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1688",
"journal-title": "J. Infect. Dis.",
"key": "ref_39",
"volume": "221",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2007.02.012",
"article-title": "Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant)",
"author": "Bork",
"doi-asserted-by": "crossref",
"first-page": "1497",
"journal-title": "J Allergy Clin. Immunol.",
"key": "ref_40",
"volume": "119",
"year": "2007"
},
{
"DOI": "10.1016/j.anai.2011.08.015",
"article-title": "Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: The FAST-3 trial",
"author": "Lumry",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "Ann. Allergy Asthma. Immunol.",
"key": "ref_41",
"volume": "107",
"year": "2011"
},
{
"DOI": "10.1111/j.1365-2249.2012.04574.x",
"article-title": "Safety and efficacy of icatibant self-administration for acute hereditary angioedema",
"author": "Bouillet",
"doi-asserted-by": "crossref",
"first-page": "303",
"journal-title": "Clin. Exp. Immunol.",
"key": "ref_42",
"volume": "168",
"year": "2012"
},
{
"DOI": "10.1002/cpdd.138",
"article-title": "Pharmacokinetics of single and repeat doses of icatibant",
"author": "Leach",
"doi-asserted-by": "crossref",
"first-page": "105",
"journal-title": "Clin. Pharmacol. Drug Dev.",
"key": "ref_43",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1111/all.12658",
"article-title": "Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate",
"author": "Zuraw",
"doi-asserted-by": "crossref",
"first-page": "1319",
"journal-title": "Allergy",
"key": "ref_44",
"volume": "70",
"year": "2015"
},
{
"DOI": "10.1111/trf.12501",
"article-title": "Pharmacokinetics of plasma-derived C1-esterase inhibitor after subcutaneous versus intravenous administration in subjects with mild or moderate hereditary angioedema: The PASSION study",
"author": "Cicardi",
"doi-asserted-by": "crossref",
"first-page": "1552",
"journal-title": "Transfusion",
"key": "ref_45",
"volume": "54",
"year": "2014"
},
{
"article-title": "Cinryze, a human plasma-derived c1 esterase inhibitor for prophylaxis of hereditary angioedema",
"author": "Cocchio",
"first-page": "293",
"journal-title": "Pharm. Ther.",
"key": "ref_46",
"volume": "34",
"year": "2009"
},
{
"DOI": "10.1016/S1473-3099(20)30141-9",
"article-title": "Convalescent plasma as a potential therapy for COVID-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "398",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_47",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(69)91295-1",
"article-title": "Replacement therapy in hereditary angioedema. Successful treatment of two patients with fresh frozen plasma",
"author": "Pickering",
"doi-asserted-by": "crossref",
"first-page": "326",
"journal-title": "Lancet",
"key": "ref_48",
"volume": "1",
"year": "1969"
},
{
"DOI": "10.1016/0091-6749(76)90017-8",
"article-title": "Letter: Fresh frozen plasma for prophylaxis in hereditary angioedema",
"author": "Fine",
"doi-asserted-by": "crossref",
"first-page": "624",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "ref_49",
"volume": "57",
"year": "1976"
},
{
"DOI": "10.1016/S0140-6736(97)09137-X",
"article-title": "Plasma bradykinin in angio-oedema",
"author": "Nussberger",
"doi-asserted-by": "crossref",
"first-page": "1693",
"journal-title": "Lancet",
"key": "ref_50",
"volume": "351",
"year": "1998"
},
{
"DOI": "10.1056/NEJM200208223470820",
"article-title": "Bradykinin-mediated angioedema",
"author": "Nussberger",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "N. Engl. J. Med.",
"key": "ref_51",
"volume": "347",
"year": "2002"
},
{
"DOI": "10.1007/s00330-020-06879-6",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Zhou, S., Zhu, T., Wang, Y., and Xia, L. (2020). Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur. Radiol."
},
{
"DOI": "10.1001/jamanetworkopen.2020.17708",
"article-title": "Outcomes Associated With Use of a Kinin B2 Receptor Antagonist Among Patients With COVID-19",
"author": "Kouijzer",
"doi-asserted-by": "crossref",
"first-page": "e2017708",
"journal-title": "JAMA Net. Open",
"key": "ref_53",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202003-0543OC",
"article-title": "Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study",
"author": "Du",
"doi-asserted-by": "crossref",
"first-page": "1372",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "ref_54",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1093/ajcp/aqaa062",
"article-title": "COVID-19 Autopsies, Oklahoma, USA",
"author": "Barton",
"doi-asserted-by": "crossref",
"first-page": "725",
"journal-title": "Am. J. Clin. Pathol.",
"key": "ref_55",
"volume": "153",
"year": "2020"
},
{
"DOI": "10.1111/all.14455",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Zhao, L., Zhang, Y.P., Yang, X., and Liu, X. (2020). Eosinopenia is associated with greater severity in patients with coronavirus disease 2019. Allergy."
},
{
"DOI": "10.1016/j.jaip.2018.04.031",
"article-title": "Eosinophils: Friends or Foes?",
"author": "Chusid",
"doi-asserted-by": "crossref",
"first-page": "1439",
"journal-title": "J. Allergy Clin. Immunol. Pract.",
"key": "ref_57",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.1111/j.1365-2222.2010.03484.x",
"article-title": "Eosinophils in health and disease: The LIAR hypothesis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "563",
"journal-title": "Clin. Exp. Allergy",
"key": "ref_58",
"volume": "40",
"year": "2010"
},
{
"DOI": "10.4049/jimmunol.1600787",
"article-title": "Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus",
"author": "Samarasinghe",
"doi-asserted-by": "crossref",
"first-page": "3214",
"journal-title": "J. Immunol.",
"key": "ref_59",
"volume": "198",
"year": "2017"
},
{
"DOI": "10.1186/s13223-020-00509-y",
"article-title": "Asthma and COVID-19: A systematic review",
"author": "Mendes",
"doi-asserted-by": "crossref",
"first-page": "5",
"journal-title": "Allergy Asthma Clin. Immunol.",
"key": "ref_60",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1182/blood-2016-03-705384",
"article-title": "Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism",
"author": "Bardou",
"doi-asserted-by": "crossref",
"first-page": "2423",
"journal-title": "Blood",
"key": "ref_61",
"volume": "128",
"year": "2016"
},
{
"DOI": "10.1152/physrev.00048.2010",
"article-title": "Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors",
"author": "Petho",
"doi-asserted-by": "crossref",
"first-page": "1699",
"journal-title": "Physiol. Rev.",
"key": "ref_62",
"volume": "92",
"year": "2012"
},
{
"DOI": "10.1016/S0140-6736(07)60242-6",
"article-title": "Renin-angiotensin system and cardiovascular risk",
"author": "Schmieder",
"doi-asserted-by": "crossref",
"first-page": "1208",
"journal-title": "Lancet",
"key": "ref_63",
"volume": "369",
"year": "2007"
},
{
"DOI": "10.1073/pnas.93.22.12490",
"article-title": "Cross-talk between the insulin and angiotensin signaling systems",
"author": "Velloso",
"doi-asserted-by": "crossref",
"first-page": "12490",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_64",
"volume": "93",
"year": "1996"
},
{
"DOI": "10.1056/NEJMcibr022472",
"article-title": "Angiotensin-converting enzyme 2—A new cardiac regulator",
"author": "Boehm",
"doi-asserted-by": "crossref",
"first-page": "1795",
"journal-title": "N. Engl. J. Med.",
"key": "ref_65",
"volume": "347",
"year": "2002"
},
{
"DOI": "10.1177/1470320313498631",
"article-title": "Angiotensin-(1-9) enhances stasis-induced venous thrombosis in the rat because of the impairment of fibrinolysis",
"author": "Mogielnicki",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "J. Renin. Angiotensin. Aldosterone Syst.",
"key": "ref_66",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.1016/j.ejmech.2019.01.040",
"article-title": "Plasma kallikrein cleaves and inactivates apelin-17: Palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents",
"author": "Fischer",
"doi-asserted-by": "crossref",
"first-page": "119",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_67",
"volume": "166",
"year": "2019"
}
],
"reference-count": 67,
"references-count": 67,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.08.11.20167353",
"id-type": "doi"
}
],
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.739580619.793585687",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/13/2/309"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Safety and Outcomes Associated with the Pharmacological Inhibition of the Kinin–Kallikrein System in Severe COVID-19",
"type": "journal-article",
"volume": "13"
}