Pilot phase results of a prospective, randomized controlled trial of narrowband ultraviolet B phototherapy in hospitalized COVID-19 patients
et al., Experimental Dermatology, doi:10.1111/exd.14617, NCT04818970, May 2022
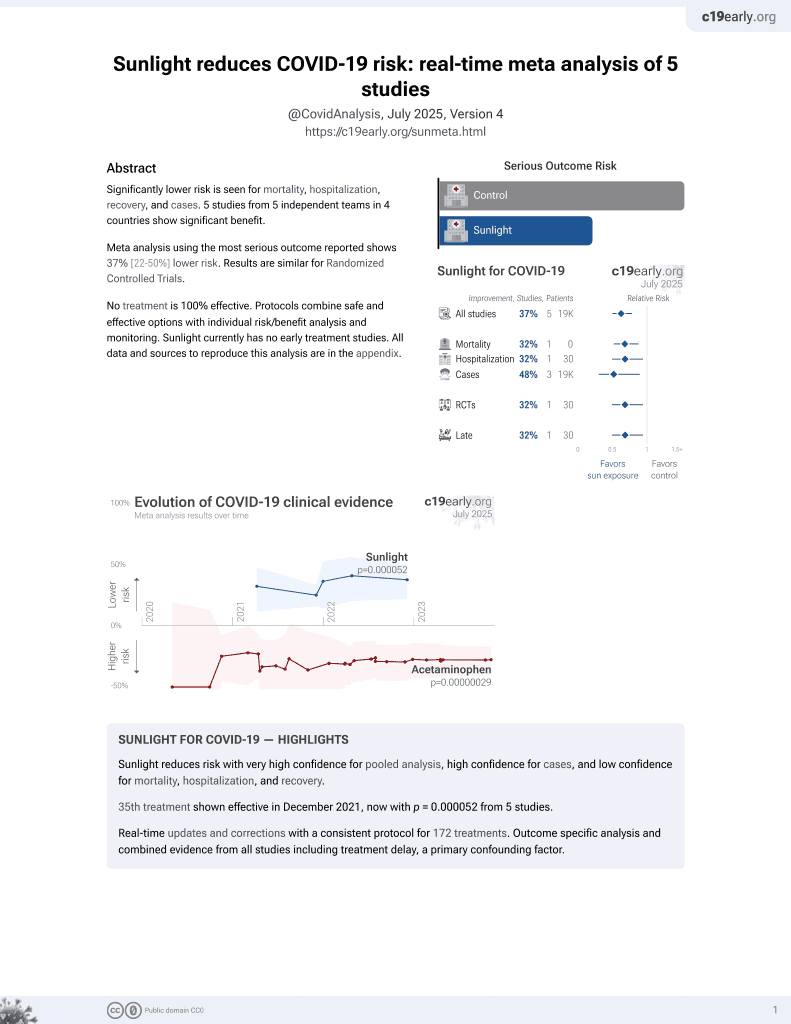
Sunlight for COVID-19
36th treatment shown to reduce risk in
December 2021, now with p = 0.000052 from 5 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 30 hospitalized COVID-19 patients showing a non-significant reduction in 28-day mortality with narrowband ultraviolet B (NB-UVB) phototherapy. This double-blind, placebo-controlled trial enrolled high-risk patients aged 50-95 with oxygen saturation <94% and at least one comorbidity. Patients received daily escalating doses of NB-UVB or placebo phototherapy on 27% of their body surface area for up to 8 consecutive days. No phototherapy-related adverse events occurred in either group. Serum vitamin D levels decreased in the treatment group contrary to expectations, suggesting vitamin D may be consumed during NB-UVB-driven response to COVID-19.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 60.0% lower, RR 0.40, p = 0.39, higher sunlight exposure 2 of 15 (13.3%), lower sunlight exposure 5 of 15 (33.3%), NNT 5.0.
|
|
risk of mechanical ventilation, no change, RR 1.00, p = 1.00, higher sunlight exposure 4 of 15 (26.7%), lower sunlight exposure 4 of 15 (26.7%).
|
|
risk of ICU admission, 25.0% higher, RR 1.25, p = 1.00, higher sunlight exposure 5 of 15 (33.3%), lower sunlight exposure 4 of 15 (26.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lau et al., 31 May 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 8 authors, study period 24 May, 2021 - 16 August, 2021, trial NCT04818970 (history).
Contact: frankhlau@gmail.com.
Pilot phase results of a prospective, randomized controlled trial of narrowband ultraviolet B phototherapy in hospitalized COVID ‐19 patients
Experimental Dermatology, doi:10.1111/exd.14617
COVID-19 morbidity and mortality are driven by poor immune regulation. Narrowband ultraviolet B (NB-UVB) phototherapy is standard of care in a number of immunedysregulated diseases. To assess the efficacy of NB-UVB phototherapy for improving COVID-19 outcomes in high-risk, hospitalized, we developed the Adaptive Photo-Protection Trial. This is a multi-center, prospective, double-blinded, randomized, placebo-controlled trial. The pilot phase results are reported here. Consecutive patients admitted with a positive COVID-19 PCR were screened for eligibility. Enrolled subjects were computer randomized 1:1 to NB-UVB or placebo phototherapy. Subjects were treated daily with escalating doses on 27% of their body surface area for up to 8 consecutive days. Primary outcomes were safety and efficacy, defined as persistent or painful erythema and 28-day mortality. Comparisons were made via non-parametric exact tests. Patients in treatment (n = 15) and placebo (n = 15) arms had similar demographics. No adverse events occurred. Twenty eight-day mortality was 13.3% in treatment vs. 33.3% in placebo arms (p = 0.39). NB-UVB phototherapy in hospitalized COVID-19 patients was safe. Decreased mortality was observed in treated patients but this was statistically non-significant. Given its low-cost, scalability, and adjunctive nature, NB-UVB has the potential to improve COVID-19 outcomes. Continuation of this trial is warranted.
| 1115 LAU et AL.
| Limitations of this study As the pilot phase of a larger clinical trial, this study was underpowered to detect statistically significant differences in clinical outcomes between treatment arms. The statistical power for a Fisher's exact test with 15 patients per group given the rates of 28-day mortality observed in this pilot is 14.1%. This power calculation will be used to refine the biostatistical considerations for the planned, larger clinical trial.
ACK N OWLED G EM ENT None.
S U PP O RTI N G I N FO R M ATI O N Additional supporting information may be found in the online version of the article at the publisher's website. Table S1 . Cytokind meridien treatment regimen. Provided initial clinical dose in accordance with the patient's Fitzpatrick Skin Type. The escalating daily dose allows for a net increase of 10% dose by accounting for the residual dose from the previous treatments.
Table S2. Baseline
References
Abhimanyu, Coussens, The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease, Photochem Photobiol Sci
Elmets, Lim, Stoff, Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy, J Am Acad Dermatol
Gorman, Weller, Investigating the potential for ultraviolet light to modulate morbidity and mortality from COVID-19: A narrative review and update, Front Cardiovasc Med
Haddad, Matsuoka, Hollis, Hu, Wortsman, Human plasma transport of vitamin D after its endogenous synthesis, J Clin Invest
Hart, Norval, More than effects in skin: ultraviolet radiationinduced changes in immune cells in human blood, Front Immunol
Iyama, Murase, Sato, Narrowband ultraviolet B phototherapy ameliorates acute graft-versus-host disease by a mechanism involving in vivo expansion of CD4 + CD25 + Foxp3+ regulatory T cells, Int J Hematol
Jager, Schöpe, Wagenpfeil, The impact of UV-dose, body surface area exposed and other factors on cutaneous vitamin D synthesis measured as serum 25(OH)D concentration: systematic review and meta-analysis, Anticancer Res
Lau, Majumder, Torabi, Vitamin D insufficiency is prevalent in severe COVID-19, medRxiv
Merzon, Tworowski, Gorohovski, Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J
Moore, Waheed, Burns, Rule of nines
Ponda, Liang, Kim, A randomized clinical trial in vitamin Ddeficient adults comparing replenishment with oral vitamin D3 with narrow-band UV type B light: effects on cholesterol and the transcriptional profiles of skin and blood, Am J Clin Nutr
Qayyum, Mohammad, Slominski, Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Am J Physiol Endocrinol Metab
Schweintzger, Gruber-Wackernagel, Shirsath, Quehenberger, Obermayer-Pietsch et al., Influence of the season on vitamin D levels and regulatory T cells in patients with polymorphic light eruption, Photochem Photobiol Sci
Slominski, Chaiprasongsuk, Janjetovic, Photoprotective properties of vitamin D and Lumisterol hydroxyderivatives, Cell Biochem Biophys
Slominski, Tuckey, Manna, Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders, Genes Immun
Slominski, Zmijewski, Plonka, Szaflarski, Paus, How UV light touches the brain and endocrine system through skin, and why, Endocrinology
Stroehlein, Wallqvist, Iannizzi, Vitamin D supplementation for the treatment of COVID-19: a living systematic review, Cochrane Database Syst Rev
Van Der Poll, De Jonge, Cate, Cytokines as regulators of coagulation. Madame curie Bioscience Database
Vieyra-Garcia, Wolf, A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer, Pharmacol Therap
Yu, Wolf, How it works: the immunology underlying phototherapy, Dermatol Clin
DOI record:
{
"DOI": "10.1111/exd.14617",
"ISSN": [
"0906-6705",
"1600-0625"
],
"URL": "http://dx.doi.org/10.1111/exd.14617",
"alternative-id": [
"10.1111/exd.14617"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-02-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-05-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-06-13"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Surgery Louisiana State University Health Sciences Center New Orleans New Orleans Louisiana USA"
}
],
"family": "Lau",
"given": "Frank H.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Surgery Louisiana State University Health Sciences Center New Orleans New Orleans Louisiana USA"
}
],
"family": "Powell",
"given": "Catherine E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine Louisiana State University Health Sciences Center New Orleans New Orleans Louisiana USA"
}
],
"family": "Adonecchi",
"given": "Giacomo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Public Health Louisiana State University Health Sciences Center New Orleans New Orleans Louisiana USA"
}
],
"family": "Danos",
"given": "Denise M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor College of Medicine Global TB program and William T Shearer Center for Human Immunobiology Houston Texas USA"
}
],
"family": "DiNardo",
"given": "Andrew R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "West Jefferson Medical Center Marrero Louisiana USA"
}
],
"family": "Chugden",
"given": "Robert J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Dermatology Medical University of Graz Graz Austria"
}
],
"family": "Wolf",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "New York Dermatology Group; Department of Dermatology Icahn School of Medicine at Mount Sinai New York New York USA"
}
],
"family": "Castilla",
"given": "Carmen F.",
"sequence": "additional"
}
],
"container-title": "Experimental Dermatology",
"container-title-short": "Experimental Dermatology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
31
]
],
"date-time": "2022-05-31T07:59:11Z",
"timestamp": 1653983951000
},
"deposited": {
"date-parts": [
[
2022,
10,
21
]
],
"date-time": "2022-10-21T10:17:11Z",
"timestamp": 1666347431000
},
"indexed": {
"date-parts": [
[
2022,
11,
18
]
],
"date-time": "2022-11-18T08:56:39Z",
"timestamp": 1668761799659
},
"is-referenced-by-count": 4,
"issue": "7",
"issued": {
"date-parts": [
[
2022,
6,
13
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2022,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
13
]
],
"date-time": "2022-06-13T00:00:00Z",
"timestamp": 1655078400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
13
]
],
"date-time": "2022-06-13T00:00:00Z",
"timestamp": 1655078400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/exd.14617",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/exd.14617",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/exd.14617",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1109-1115",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
6,
13
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
13
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.3389/fcvm.2020.616527",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1016/j.pharmthera.2020.107784",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1016/j.det.2019.08.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"article-title": "Vitamin D insufficiency is prevalent in severe COVID‐19",
"author": "Lau FH",
"journal-title": "medRxiv",
"key": "e_1_2_10_5_1",
"year": "2020"
},
{
"article-title": "Vitamin D supplementation for the treatment of COVID‐19: a living systematic review",
"author": "Stroehlein JK",
"first-page": "CD015043",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_2_10_6_1",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/j.jaad.2019.04.042",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"article-title": "The impact of UV‐dose, body surface area exposed and other factors on cutaneous vitamin D synthesis measured as serum 25(OH)D concentration: systematic review and meta‐analysis",
"author": "Jager N",
"first-page": "1165",
"issue": "2",
"journal-title": "Anticancer Res",
"key": "e_1_2_10_8_1",
"volume": "38",
"year": "2018"
},
{
"author": "Moore RA",
"key": "e_1_2_10_9_1",
"volume-title": "StatPearls.",
"year": "2021"
},
{
"key": "e_1_2_10_10_1",
"unstructured": "Evaluation of efficacy duration of remission and safety of a light and occlusive patch. therapy for plaque psoriasis. March 15 2022.https://clinicaltrials.gov/ct2/show/NCT03180866"
},
{
"DOI": "10.3945/ajcn.116.150367",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1039/C5PP00398A",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1172/JCI116492",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1152/ajpendo.00174.2021",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1007/s12013-020-00913-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1038/s41435-020-0096-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1039/C6PP00355A",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1210/en.2017-03230",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.3389/fimmu.2021.694086",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1007/s12185-014-1530-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"author": "Poll T",
"key": "e_1_2_10_22_1",
"volume-title": "Madame curie Bioscience Database",
"year": "2013"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/exd.14617"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Dermatology",
"Molecular Biology",
"Biochemistry"
],
"subtitle": [],
"title": "Pilot phase results of a prospective, randomized controlled trial of narrowband ultraviolet B phototherapy in hospitalized\n <scp>COVID</scp>\n ‐19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "31"
}