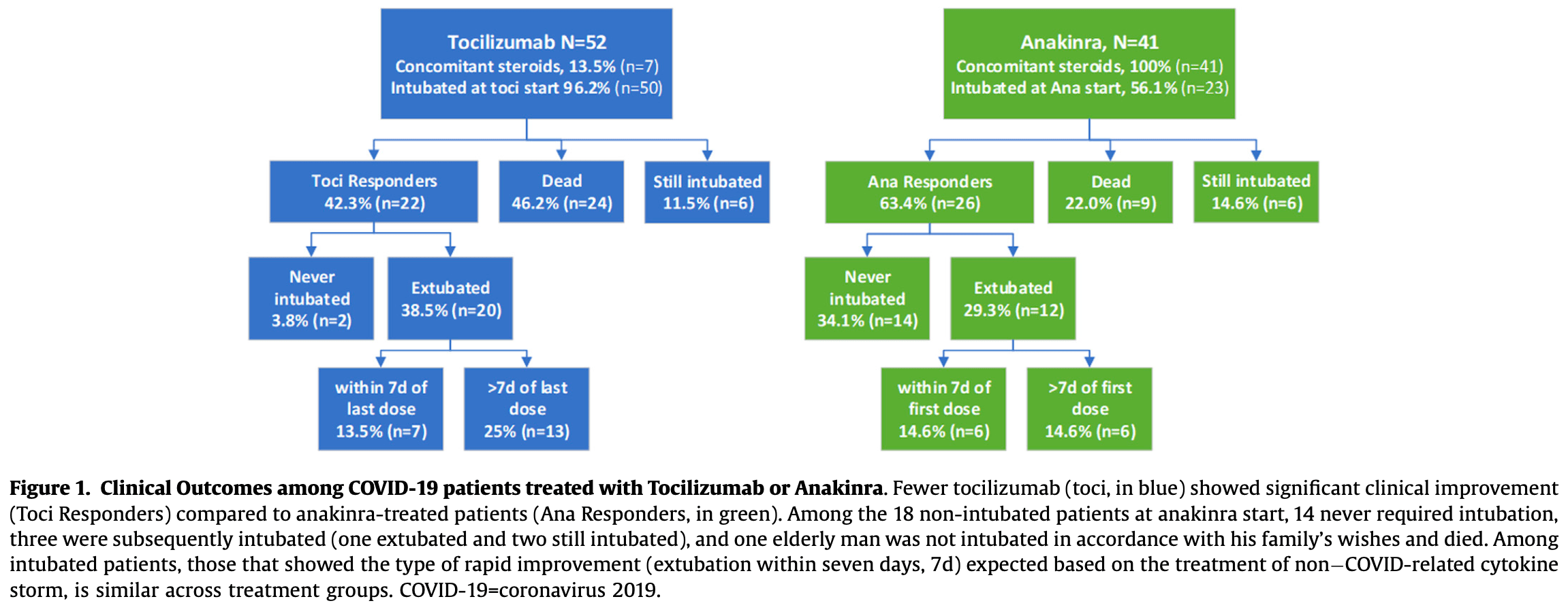
Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.07.081, Oct 2020
Retrospective 93 hospitalized COVID-19 patients with cytokine storm showing no significant difference between anakinra and tocilizumab treatment after adjusting for baseline characteristics.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 117.4% higher, HR 2.17, p = 0.53, treatment 24 of 52 (46.2%), control 9 of 41 (22.0%), inverted to make HR<1 favor treatment, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Langer-Gould et al., 31 Oct 2020, retrospective, USA, peer-reviewed, 8 authors, study period 1 March, 2020 - 30 April, 2020, average treatment delay 14.0 days, this trial compares with another treatment - results may be better when compared to placebo.
Contact: annette.m.langer-gould@kp.org.
Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.07.081
Objective: To examine outcomes among patients who were treated with the targeted anti-cytokine agents, anakinra or tocilizumab, for COVID-19 -related cytokine storm (COVID19-CS). Methods: We conducted a retrospective cohort study of all SARS-coV2-RNA-positive patients treated with tocilizumab or anakinra in Kaiser Permanente Southern California. Local experts developed and implemented criteria to define COVID19-CS. All variables were extracted from electronic health records. Results: At tocilizumab initiation (n = 52), 50 (96.2%) were intubated, and only seven (13.5%) received concomitant corticosteroids. At anakinra initiation (n = 41), 23 (56.1%) were intubated, and all received concomitant corticosteroids. Fewer anakinra-treated patients died (n = 9, 22%) and more were extubated/ never intubated (n = 26, 63.4%) compared to tocilizumab-treated patients (n = 24, 46.2% dead, n = 22, 42.3% extubated/never intubated). Patients who died had more severe sepsis and respiratory failure and met COVID-CS laboratory criteria longer (median = 3 days) compared to those extubated/never intubated (median = 1 day). After accounting for differences in disease severity at treatment initiation, this apparent superiority of anakinra over tocilizumab was no longer statistically significant (propensity scoreadjusted hazards ratio 0.46, 95% confidence interval 0.18-1.20). Conclusions: Prompt identification and treatment of COVID19-CS before intubation may be more important than the specific type of anti-inflammatory treatment. Randomized controlled trials of targeted anti-cytokine treatments and corticosteroids should report the duration of cytokine storm in addition to clinical severity at randomization.
Author contributions Annette Langer-Gould conceptualized and designed the study, collected and interpreted the data, drafted and revised the manuscript for intellectual content, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analyses. Jessica B. Smith collected, analyzed, and interpreted the data and revised the manuscript for content. Edlin G. Gonzales collected the data and revised the manuscript for content. Rhina D. Castillo conceptualized and designed the study and revised the manuscript for content. Judith G. Figueroa conceptualized and designed the study and revised the manuscript for content. Anusha Ramanathan conceptualized and designed the study and revised the manuscript for content. Bonnie H. Li analyzed and interpreted the data and revised the manuscript for content. Michael K. Gould conceptualized and designed the study and revised the manuscript for content.
Conflicts of interests Annette Langer-Gould currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from ICER. Michael K. Gould has received research support through his employer from Medial EarlySign to develop computer models of lung cancer risk and royalties from UpToDate to co-author topics on lung cancer diagnosis and staging. Jessica B. Smith, Edlin G. Gonzales, Rhina D...
References
Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting potential drivers of COVID-19: Neutrophil extracellular traps, J Exp Med, doi:10.1084/jem.20200652
Cavalli, Luca, Campochiaro, Della-Torre, Ripa et al., Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(20)30127-2
Halyabar, Chang, Schoettler, Schwartz, Baris et al., Calm in the midst of cytokine storm: a collaborative approach to the diagnosis and treatment of hemophagocytic lymphohistiocytosis and macrophage activation syndrome, Pediatr Rheumatol Online J, doi:10.1186/s12969-019-0309-6
Jordan, Allen, Greenberg, Henry, Hermiston et al., Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO, Pediatr Blood Cancer, doi:10.1002/pbc.27929
Koebnick, Langer-Gould, Gould, Chao, Iyer et al., Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data, Perm J, doi:10.7812/tpp/12-031
Lee, Gardner, Porter, Louis, Ahmed et al., Current concepts in the diagnosis and management of cytokine release syndrome, Blood, doi:10.1182/blood-2014-05-552729
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Toniati, Piva, Cattalini, Garrafa, Regola et al., Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy, Autoimmun Rev, doi:10.1016/j.autrev.2020.102568
Xu, Han, Li, Sun, Wang et al., Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2005615117
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1016/j.ijid.2020.07.081",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2020.07.081",
"alternative-id": [
"S1201971220306093"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2020.07.081"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Southern California Permanente Medical Group. Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Langer-Gould",
"given": "Annette",
"sequence": "first"
},
{
"affiliation": [],
"family": "Smith",
"given": "Jessica B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonzales",
"given": "Edlin G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castillo",
"given": "Rhina D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Figueroa",
"given": "Judith Garza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramanathan",
"given": "Anusha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Bonnie H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gould",
"given": "Michael K.",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ijidonline.com",
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
8,
6
]
],
"date-time": "2020-08-06T01:54:20Z",
"timestamp": 1596678860000
},
"deposited": {
"date-parts": [
[
2020,
10,
24
]
],
"date-time": "2020-10-24T00:43:50Z",
"timestamp": 1603500230000
},
"indexed": {
"date-parts": [
[
2024,
9,
16
]
],
"date-time": "2024-09-16T10:37:52Z",
"timestamp": 1726483072696
},
"is-referenced-by-count": 72,
"issued": {
"date-parts": [
[
2020,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
29
]
],
"date-time": "2020-07-29T00:00:00Z",
"timestamp": 1595980800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971220306093?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971220306093?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "291-297",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1084/jem.20200652",
"article-title": "Targeting potential drivers of COVID-19: Neutrophil extracellular traps",
"author": "Barnes",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "J Exp Med",
"key": "10.1016/j.ijid.2020.07.081_bib0005",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"first-page": "e325",
"issue": "6",
"journal-title": "Lancet Rheumatol",
"key": "10.1016/j.ijid.2020.07.081_bib0010",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1186/s12969-019-0309-6",
"article-title": "Calm in the midst of cytokine storm: a collaborative approach to the diagnosis and treatment of hemophagocytic lymphohistiocytosis and macrophage activation syndrome",
"author": "Halyabar",
"doi-asserted-by": "crossref",
"first-page": "7",
"issue": "1",
"journal-title": "Pediatr Rheumatol Online J",
"key": "10.1016/j.ijid.2020.07.081_bib0015",
"volume": "17",
"year": "2019"
},
{
"DOI": "10.1002/pbc.27929",
"article-title": "Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO",
"author": "Jordan",
"doi-asserted-by": "crossref",
"first-page": "e27929",
"issue": "11",
"journal-title": "Pediatr Blood Cancer",
"key": "10.1016/j.ijid.2020.07.081_bib0020",
"volume": "66",
"year": "2019"
},
{
"DOI": "10.7812/TPP/12-031",
"article-title": "Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data",
"author": "Koebnick",
"doi-asserted-by": "crossref",
"first-page": "37",
"issue": "3",
"journal-title": "Perm J",
"key": "10.1016/j.ijid.2020.07.081_bib0025",
"volume": "16",
"year": "2012"
},
{
"DOI": "10.1182/blood-2014-05-552729",
"article-title": "Current concepts in the diagnosis and management of cytokine release syndrome",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "188",
"issue": "2",
"journal-title": "Blood",
"key": "10.1016/j.ijid.2020.07.081_bib0030",
"volume": "124",
"year": "2014"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID-19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1033",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2020.07.081_bib0035",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"article-title": "Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy",
"author": "Toniati",
"doi-asserted-by": "crossref",
"first-page": "102568",
"issue": "7",
"journal-title": "Autoimmun Rev",
"key": "10.1016/j.ijid.2020.07.081_bib0040",
"volume": "19",
"year": "2020"
},
{
"author": "University of Oxford",
"key": "10.1016/j.ijid.2020.07.081_bib0045",
"series-title": "RECOVERY: Randomised Evaluation of COVID-19 Therapy",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "10970",
"issue": "20",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.ijid.2020.07.081_bib0050",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2020.07.081_bib0055",
"volume": "395",
"year": "2020"
}
],
"reference-count": 11,
"references-count": 11,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971220306093"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "99"
}