Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19
et al., Annals of Internal Medicine, doi:10.7326/M22-1286, Apr 2023
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 2,571 patients treated with mAbs in the USA, and 5,135 control patients, showing lower combined mortality/hospitalization for bamlanivimab, bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, and bebtelovimab, with statistical significance only for casirivimab/imdevimab.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending sotrovimab also recommended them, or
because the patient seeking out sotrovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.14-6, BA.4, BA.57, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.18, and no efficacy for BA.29, XBB, XBB.1.5, ХВВ.1.9.110, XBB.1.16, BQ.1.1.45, and CL.18. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments11.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death/hospitalization, 30.0% lower, RR 0.70, p = 0.14, treatment 22 of 500 (4.4%), control 63 of 999 (6.3%), NNT 52, delta and omicron variants, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
8.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
9.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Kip et al., 4 Apr 2023, retrospective, USA, peer-reviewed, 16 authors, study period 8 December, 2020 - 31 August, 2022.
Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19
Annals of Internal Medicine, doi:10.7326/m22-1286
Background: Treatment guidelines and U.S. Food and Drug Administration emergency use authorizations (EUAs) of monoclonal antibodies (mAbs) for treatment of high-risk outpatients with mild to moderate COVID-19 changed frequently as different SARS-CoV-2 variants emerged. Objective: To evaluate whether early outpatient treatment with mAbs, overall and by mAb product, presumed SARS-CoV-2 variant, and immunocompromised status, is associated with reduced risk for hospitalization or death at 28 days. Design: Hypothetical pragmatic randomized trial from observational data comparing mAb-treated patients with a propensity score-matched, nontreated control group. Setting: Large U.S. health care system. Participants: High-risk outpatients eligible for mAb treatment under any EUA with a positive SARS-CoV-2 test result from 8 December 2020 to 31 August 2022. Intervention: Single-dose intravenous mAb treatment with bamlanivimab, bamlanivimab-etesevimab, sotrovimab, bebtelovimab, or intravenous or subcutaneous casirivimab-imdevimab administered within 2 days of a positive SARS-CoV-2 test result. Measurements: The primary outcome was hospitalization or death at 28 days among treated patients versus a nontreated control group (no treatment or treatment ≥3 days after SARS-CoV-2 test date).
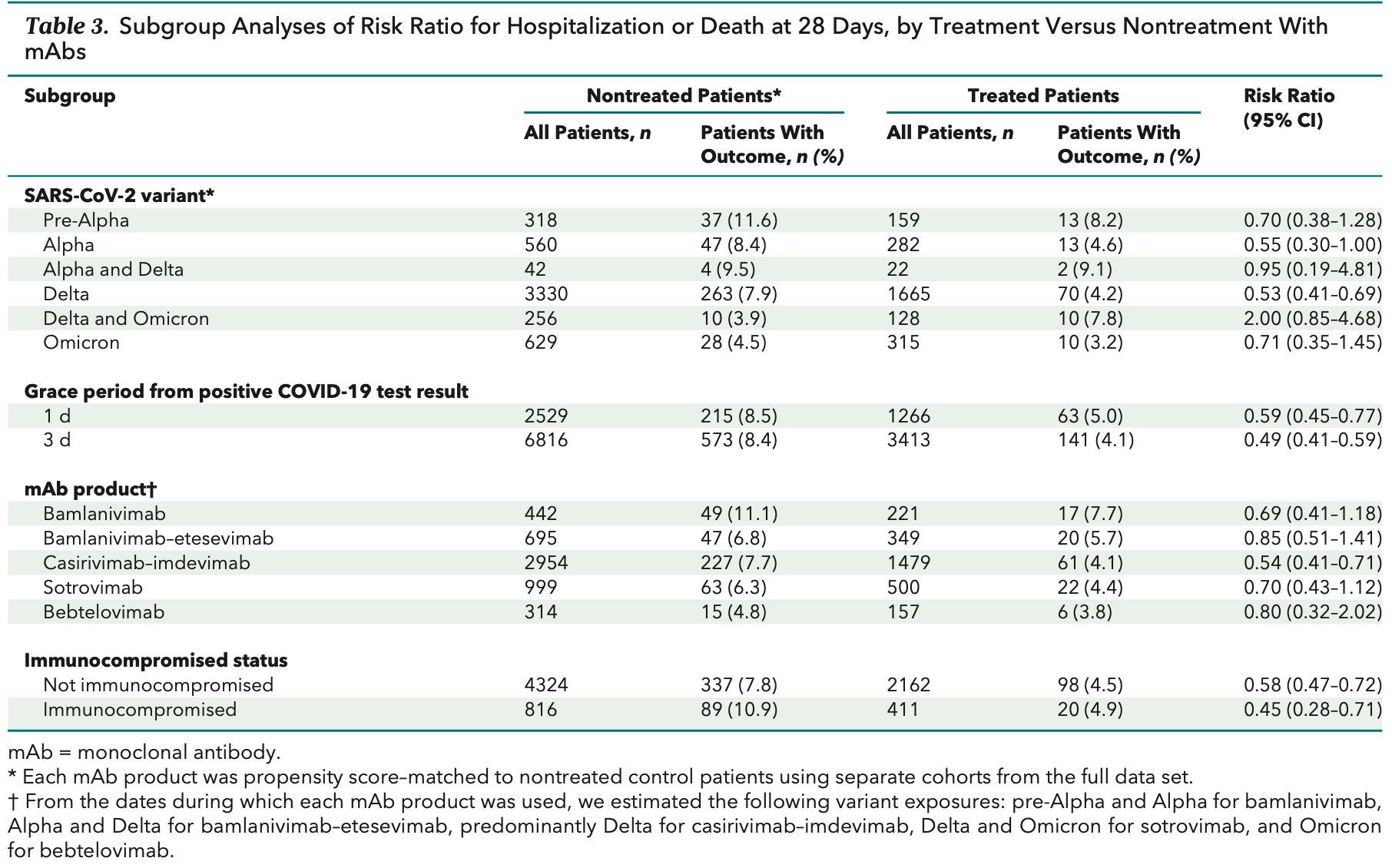
Results: The risk for hospitalization or death at 28 days was 4.6% in 2571 treated patients and 7.6% in 5135 nontreated control patients (risk ratio [RR], 0.61 [95% CI, 0.50 to 0.74]). In sensitivity analyses, the corresponding RRs for 1-and 3-day treatment grace periods were 0.59 and 0.49, respectively. In subgroup analyses, those receiving mAbs when the Alpha and Delta variants were presumed to be predominant had estimated RRs of 0.55 and 0.53, respectively, compared with 0.71 for the Omicron variant period. Relative risk estimates for individual mAb products all suggested lower risk for hospitalization or death. Among immunocompromised patients, the RR was 0.45 (CI, 0.28 to 0.71). Limitations: Observational study design, SARS-CoV-2 variant presumed by date rather than genotyping, no data on symptom severity, and partial data on vaccination status.
Conclusion: Early mAb treatment among outpatients with COVID-19 is associated with lower risk for hospitalization or death for various mAb products and SARS-CoV-2 variants.
The bottom section depicts 1:2 propensity score matching of treated and nontreated patients. mAb = monoclonal antibody.
References
Ahmed, Quadeer, Mckay, Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies, Viruses, doi:10.3390/v12030254
Austin, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav Res
Austin, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharm Stat, doi:10.1002/pst.433
Bariola, Mccreary, Wadas, Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection, Open Forum Infect Dis, doi:10.1093/ofid/ofab254
Chen, Nirula, Heller, BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Cox, Peacock, Harvey, COVID-19 Genomics UK (COG-UK) Consortium. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies, Nat Rev Microbiol, doi:10.1038/s41579-022-00809-7
Dryden-Peterson, Kim, Joyce, Bebtelovimab for high-risk outpatients with early COVID-19 in a large US health system, Open Forum Infect Dis, doi:10.1093/ofid/ofac565
Focosi, Mcconnell, Casadevall, The Omicron variant of concern: diversification and convergent evolution in spike protein, and escape from anti-spike monoclonal antibodies, Drug Resist Updat, doi:10.1016/j.drup.2022.100882
Food, not currently authorized for emergency use in the U
Gil, Ginex, Maestro, COVID-19: drug targets and potential treatments, J Med Chem, doi:10.1021/acs.jmedchem.0c00606
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Gupta, Vemula, Donde, In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1751300
Haidar, Agha, Bilderback, Prospective evaluation of coronavirus disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 Vaccination in the Immunocompromised Study (COVICS), Clin Infect Dis, doi:10.1093/cid/ciac103
Huang, Mccreary, Bariola, Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge: a cohort study and randomized comparative effectiveness trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20957
Lewnard, Hong, Patel, Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California, doi:10.1038/s41591-022-01887-z
Lin, Hung, Lai, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: a systematic review and meta-analysis of randomized controlled trials, J Med Virol, doi:10.1002/jmv.27623
Liu, Wang, Nair, Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike, Nature, doi:10.1038/s41586-020-2571-7
Lloyd, Gandhi, Petty, Monoclonal antibodies for COVID-19, JAMA, doi:10.1001/jama.2021.1225
Mccreary, Bariola, Minnier, The comparative effectiveness of COVID-19 monoclonal antibodies: a learning health system randomized clinical trial, Contemp Clin Trials, doi:10.1016/j.cct.2022.106822
Mccreary, Bariola, Wadas, Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.6920
Mccreary, Kip, Bariola, A learning health system approach to the COVID-19 pandemic: system-wide changes in clinical practice and 30-day mortality among hospitalized patients, Learn Health Sys, doi:10.1002/lrh2.10304
Mccreary, Kip, Collins, Evaluation of bebtelovimab for treatment of Covid-19 during the SARS-CoV-2 Omicron variant era, Open Forum Infect Dis, doi:10.1093/ofid/ofac517
Mehta, Goel, Kabarriti, Case fatality rate of cancer patients with COVID-19 in a New York hospital system, Cancer Discov, doi:10.1158/2159-8290.CD-20-0516
Nagler, Horwitz, Jones, The impact of COVID-19 monoclonal antibodies on clinical outcomes: a retrospective cohort study, Am J Health Syst Pharm, doi:10.1093/ajhp/zxac295
Nathan, Shawa, De, Torre, A narrative review of the clinical practicalities of bamlanivimab and etesevimab antibody therapies for SARS-CoV-2, Infect Dis Ther, doi:10.1007/s40121-021-00515-6
Reitz, Seymour, Vates, Strategies to Promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients, BMJ Open, doi:10.1136/bmjopen-2020-037690
Rosenbaum, Db, The central role of the propensity score in observational studies for causal effects, Biometrika, doi:10.1093/biomet/70.1.41
Services, COVID-19 Monoclonal Antibodies
Shang, Wan, Luo, Cell entry mechanisms of SARS-CoV-2, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2003138117
Stokes, Zambrano, Anderson, Coronavirus disease 2019 case surveillance -United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6924e2
Tao, Tzou, Pond, Susceptibility of SARS-CoV-2 Omicron variants to therapeutic monoclonal antibodies: systematic review and meta-analysis, Microbiol Spectr, doi:10.1128/spectrum.00926-22
Wang, Casner, Nair, A monoclonal antibody that neutralizes SARS-CoV-2 variants, SARS-CoV, and other sarbecoviruses, Emerg Microbes Infect, doi:10.1080/22221751.2021.2011623
Weinreich, Sivapalasingam, Norton, Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Widyasari, Kim, A review of the currently available antibody therapy for the treatment of coronavirus disease 2019 (COVID-19), Antibodies, doi:10.3390/antib12010005
Wynia, Beaty, Bennett, Real-world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients, Chest, doi:10.1016/j.chest.2022.10.020
DOI record:
{
"DOI": "10.7326/m22-1286",
"ISSN": [
"0003-4819",
"1539-3704"
],
"URL": "http://dx.doi.org/10.7326/M22-1286",
"alternative-id": [
"10.7326/M22-1286"
],
"author": [
{
"affiliation": [
{
"name": "Clinical Analytics, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (K.E.K., K.C., W.G., J.C.M., O.C.M.)"
}
],
"family": "Kip",
"given": "Kevin E.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (E.K.M., G.M.S., J.R.B.)"
}
],
"family": "McCreary",
"given": "Erin K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Analytics, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (K.E.K., K.C., W.G., J.C.M., O.C.M.)"
}
],
"family": "Collins",
"given": "Kevin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6149-0424",
"affiliation": [
{
"name": "Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (T.E.M., P.L.K.)"
}
],
"authenticated-orcid": false,
"family": "Minnier",
"given": "Tami E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5562-8880",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (E.K.M., G.M.S., J.R.B.)"
}
],
"authenticated-orcid": false,
"family": "Snyder",
"given": "Graham M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5141-6239",
"affiliation": [
{
"name": "Clinical Analytics, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (K.E.K., K.C., W.G., J.C.M., O.C.M.)"
}
],
"authenticated-orcid": false,
"family": "Garrard",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Analytics, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (K.E.K., K.C., W.G., J.C.M., O.C.M.)"
}
],
"family": "McKibben",
"given": "Jeffrey C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (D.M.Y., R.J.W.)"
}
],
"family": "Yealy",
"given": "Donald M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (C.W.S., D.C.A.)"
}
],
"family": "Seymour",
"given": "Christopher W.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7649-1633",
"affiliation": [
{
"name": "Department of Emergency Medicine and Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (D.T.H.)"
}
],
"authenticated-orcid": false,
"family": "Huang",
"given": "David T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (E.K.M., G.M.S., J.R.B.)"
}
],
"family": "Bariola",
"given": "J. Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (M.S.)."
}
],
"family": "Schmidhofer",
"given": "Mark",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6152-9990",
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (D.M.Y., R.J.W.)"
}
],
"authenticated-orcid": false,
"family": "Wadas",
"given": "Richard J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7026-5181",
"affiliation": [
{
"name": "Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (C.W.S., D.C.A.)"
}
],
"authenticated-orcid": false,
"family": "Angus",
"given": "Derek C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8337-4777",
"affiliation": [
{
"name": "Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (T.E.M., P.L.K.)"
}
],
"authenticated-orcid": false,
"family": "Kip",
"given": "Paula L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Analytics, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania (K.E.K., K.C., W.G., J.C.M., O.C.M.)"
}
],
"family": "Marroquin",
"given": "Oscar C.",
"sequence": "additional"
}
],
"container-title": "Annals of Internal Medicine",
"container-title-short": "Ann Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
3
]
],
"date-time": "2023-04-03T21:00:29Z",
"timestamp": 1680555629000
},
"deposited": {
"date-parts": [
[
2023,
4,
17
]
],
"date-time": "2023-04-17T21:02:01Z",
"timestamp": 1681765321000
},
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T19:13:42Z",
"timestamp": 1712690022806
},
"is-referenced-by-count": 17,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"member": "4285",
"original-title": [],
"page": "496-504",
"prefix": "10.7326",
"published": {
"date-parts": [
[
2023,
4
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "American College of Physicians",
"reference": [
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "r5-M221286"
},
{
"DOI": "10.1001/jama.2021.1225",
"doi-asserted-by": "publisher",
"key": "r7-M221286"
},
{
"DOI": "10.1093/ofid/ofab254",
"doi-asserted-by": "publisher",
"key": "r8-M221286"
},
{
"DOI": "10.1093/ofid/ofac517",
"doi-asserted-by": "publisher",
"key": "r12-M221286"
},
{
"DOI": "10.1093/ofid/ofac565",
"doi-asserted-by": "publisher",
"key": "r13-M221286"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6920",
"doi-asserted-by": "publisher",
"key": "r14-M221286"
},
{
"DOI": "10.1016/j.cct.2022.106822",
"doi-asserted-by": "publisher",
"key": "r15-M221286"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20957",
"doi-asserted-by": "publisher",
"key": "r16-M221286"
},
{
"DOI": "10.1101/2021.08.26.21262661",
"doi-asserted-by": "crossref",
"key": "r17-M221286",
"unstructured": "McCreary EK, Kip KE, Bariola JR, et al. A learning health system approach to the COVID-19 pandemic: system-wide changes in clinical practice and 30-day mortality among hospitalized patients. Learn Health Sys. 2022;6:e10304. doi:10.1002/lrh2.10304"
},
{
"DOI": "10.1136/bmjopen-2020-037690",
"doi-asserted-by": "publisher",
"key": "r18-M221286"
},
{
"DOI": "10.1080/00273171.2011.568786",
"doi-asserted-by": "publisher",
"key": "r23-M221286"
},
{
"DOI": "10.1093/biomet/70.1.41",
"doi-asserted-by": "crossref",
"key": "r24-M221286",
"unstructured": "Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. doi:10.1093/biomet/70.1.41"
},
{
"DOI": "10.1002/pst.433",
"doi-asserted-by": "publisher",
"key": "r25-M221286"
},
{
"DOI": "10.1016/j.chest.2022.10.020",
"doi-asserted-by": "publisher",
"key": "r26-M221286"
},
{
"DOI": "10.1093/ajhp/zxac295",
"doi-asserted-by": "publisher",
"key": "r27-M221286"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "r28-M221286"
},
{
"DOI": "10.1001/jama.2022.2832",
"doi-asserted-by": "publisher",
"key": "r29-M221286"
},
{
"DOI": "10.1002/jmv.27623",
"doi-asserted-by": "publisher",
"key": "r30-M221286"
},
{
"DOI": "10.1158/2159-8290.CD-20-0516",
"doi-asserted-by": "publisher",
"key": "r31-M221286"
},
{
"DOI": "10.15585/mmwr.mm6924e2",
"doi-asserted-by": "publisher",
"key": "r32-M221286"
},
{
"DOI": "10.1093/cid/ciac103",
"doi-asserted-by": "publisher",
"key": "r33-M221286"
},
{
"DOI": "10.1080/07391102.2020.1751300",
"doi-asserted-by": "publisher",
"key": "r34-M221286"
},
{
"DOI": "10.3390/v12030254",
"doi-asserted-by": "publisher",
"key": "r35-M221286"
},
{
"DOI": "10.1021/acs.jmedchem.0c00606",
"doi-asserted-by": "publisher",
"key": "r36-M221286"
},
{
"DOI": "10.3390/antib12010005",
"doi-asserted-by": "publisher",
"key": "r37-M221286"
},
{
"DOI": "10.1073/pnas.2003138117",
"doi-asserted-by": "publisher",
"key": "r38-M221286"
},
{
"DOI": "10.1038/s41586-020-2571-7",
"doi-asserted-by": "publisher",
"key": "r39-M221286"
},
{
"DOI": "10.1007/s40121-021-00515-6",
"doi-asserted-by": "publisher",
"key": "r40-M221286"
},
{
"DOI": "10.1038/s41579-022-00809-7",
"doi-asserted-by": "publisher",
"key": "r41-M221286"
},
{
"DOI": "10.1128/spectrum.00926-22",
"doi-asserted-by": "publisher",
"key": "r42-M221286"
},
{
"DOI": "10.1016/j.drup.2022.100882",
"doi-asserted-by": "publisher",
"key": "r43-M221286"
},
{
"DOI": "10.1080/22221751.2021.2011623",
"doi-asserted-by": "publisher",
"key": "r44-M221286"
},
{
"DOI": "10.1038/s41591-022-01887-z",
"doi-asserted-by": "publisher",
"key": "r46-M221286"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.acpjournals.org/doi/10.7326/M22-1286"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Internal Medicine"
],
"subtitle": [
"A Cohort Study"
],
"title": "Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19",
"type": "journal-article",
"volume": "176"
}
kip
