Bebtelovimab for high-risk outpatients with early COVID-19 in a large US health system
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac565, Oct 2022
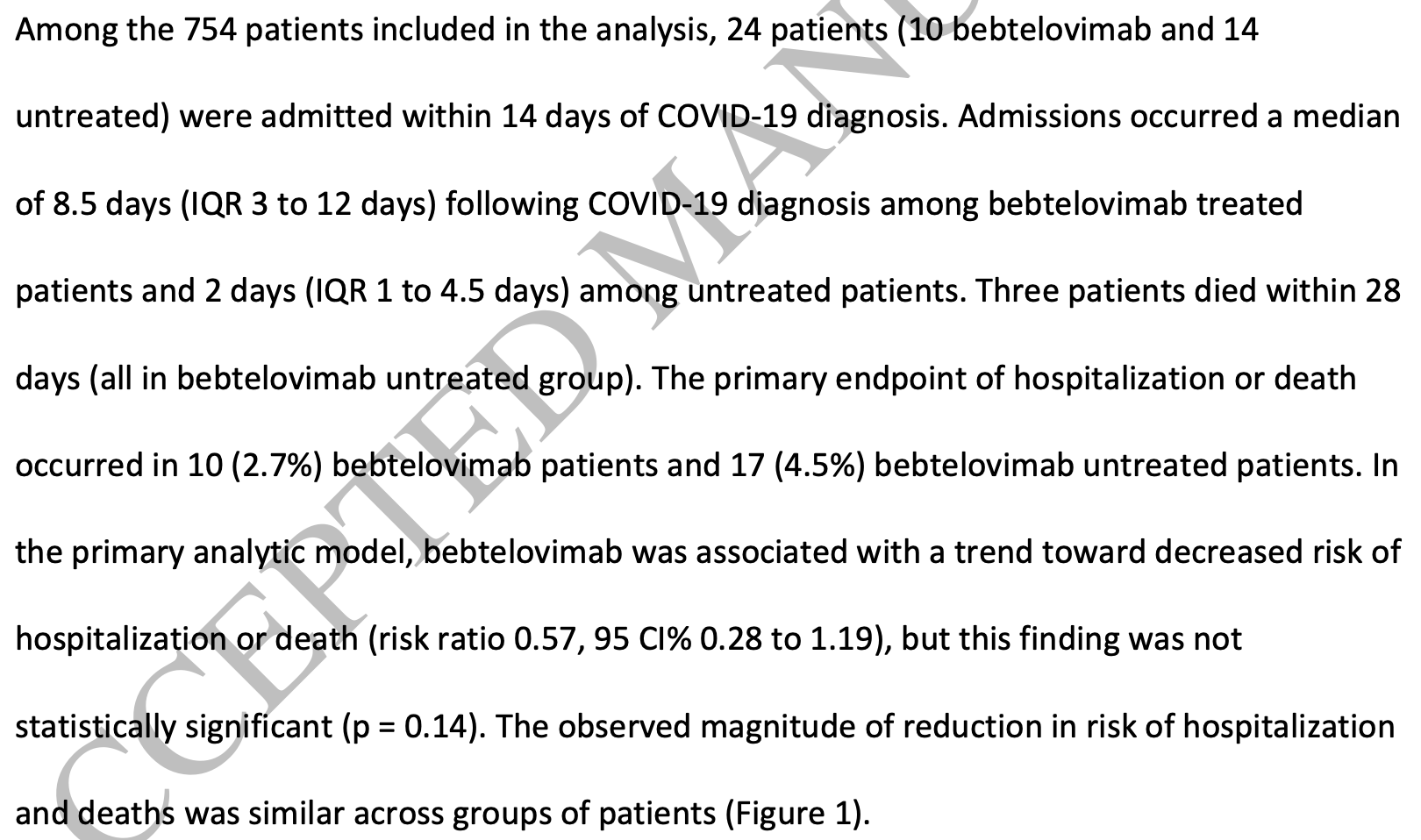
Retrospective 377 outpatients in the USA and matched controls, showing lower hospitalization/mortality with bebtelovimab treatment, without statistical significance. Notably, none of the patients that died in the control group were hospitalized within 14 days (later hospitalization is not reported). There may be a difference in the populations in terms of propensity to receive SOC.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.5, XBB.1.9.13.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments4.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 377 (0.0%), control 3 of 377 (0.8%), NNT 126, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of death/hospitalization, 43.0% lower, RR 0.57, p = 0.14, treatment 10 of 377 (2.7%), control 17 of 377 (4.5%), NNT 54.
|
|
risk of hospitalization, 28.6% lower, RR 0.71, p = 0.53, treatment 10 of 377 (2.7%), control 14 of 377 (3.7%), NNT 94.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Dryden-Peterson et al., 27 Oct 2022, retrospective, USA, peer-reviewed, 7 authors, study period 16 March, 2022 - 31 May, 2022, average treatment delay 3.0 days.
Contact: awoolley@bwh.harvard.edu, sldrydenpeterson@bwh.harvard.edu.
Bebtelovimab for high-risk outpatients with early COVID-19 in a large US health system
doi:10.1093/ofid/ofac565/6775265
There are limited data for the clinical efficacy of bebtelovimab in preventing severe COVID-19. Among outpatients unable to take nirmatrelvir-ritonavir at a large health system, 10 of 377 (2.7%) patients who received bebtelovimab and 17 of 377 (4.5%) matched untreated patients were hospitalized or died. The 43% observed risk reduction with bebtelovimab was not statistically significant (p = 0.14).
Author contributions. S.D.P. and A.E.W. designed the study. S.D.P., A.K., M.J., and A.E.W. collected and adjudicated the data. S.D.P., J.A.J., A.Y.K., L.R.B., and A.E.W. provided scientific interpretation of the data. S.D.P. and A.E.W. performed the statistical analysis. S.D.P., A.K., M.J., and A.E.W. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S.D.P. and A.E.W. drafted the manuscript. All
References
Aggarwal, Beaty, Bennett, Carlson, Ginde, Change in Effectiveness of Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients During the Omicron Phase, Medrxiv. Published, doi:10.1101/2022.06.17.22276575
Dougan, Azizad, Chen, Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19, Medrxiv. Published, doi:10.1101/2022.03.10.22272100
Dougan, Nirula, Azizad, Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, New Engl J Med, doi:10.1056/nejmoa2102685
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, Published online, doi:10.1101/2022.06.14.22276393
Ganatra, Dani, Ahmad, Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19, Clin Infect Dis Official Publ Infect Dis Soc Am. Published, doi:10.1093/cid/ciac673
Ganesh, Philpot, Bierle, Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab among High-Risk Patients with Mild to Moderate Coronavirus Disease 2019, J Infect Dis, doi:10.1093/infdis/jiab377
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2
Halekoh, Højsgaard, Yan, The R Package geepack for Generalized Estimating Equations, J Stat Softw, doi:10.18637/jss.v015.i02
Khare, Gurry, Freitas, GISAID's Role in Pandemic Response, China Cdc Wkly, doi:10.46234/ccdcw2021.255
Liang, Zeger, Longitudinal data analysis using generalized linear models, Biometrika
Razonable, Horo, Hanson, Comparable Outcomes for Bebtelovimab and Ritonavir-Boosted Nirmatrelvir Treatment in High-Risk Patients With Coronavirus Disease
Rubin, Liu, Giobbie-Hurder, Performance of a Triage Protocol for Monoclonal Antibodies in a Mixed Vaccinated and Unvaccinated Cohort of COVID-19 Patients Treated With Intravenous Infusion or Subcutaneous Injection, Open Forum Infect Dis, doi:10.1093/ofid/ofac182
Takashita, Yamayoshi, Simon, Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12, New Engl J Med. Published, doi:10.1056/nejmc2207519
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, New Engl J Med, doi:10.1056/nejmoa2035002
Yetmar, Beam, Horo, Outcomes of Bebtelovimab and Sotrovimab Treatment of Solid Organ Transplant Recipients with Mild-to-moderate COVID-19 during the Omicron Epoch, Transpl Infect Dis. Published, doi:10.1111/tid.13901
Zou, A Modified Poisson Regression Approach to Prospective Studies with Binary Data, American Journal of Epidemiology, doi:10.1093/aje/kwh090
DOI record:
{
"DOI": "10.1093/ofid/ofac565",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofac565",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>There are limited data for the clinical efficacy of bebtelovimab in preventing severe COVID-19. Among outpatients unable to take nirmatrelvir-ritonavir at a large health system, 10 of 377 (2.7%) patients who received bebtelovimab and 17 of 377 (4.5%) matched untreated patients were hospitalized or died. The 43% observed risk reduction with bebtelovimab was not statistically significant (p = 0.14).</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Brigham and Women’s Hospital , Boston, Massachusetts , USA"
},
{
"name": "Dana Farber Cancer Institute , Boston, Massachusetts , USA"
},
{
"name": "Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health , Boston, Massachusetts , USA"
},
{
"name": "Botswana Harvard AIDS Institute , Gaborone , Botswana"
},
{
"name": "Harvard Medical School , Boston, Massachusetts , USA"
}
],
"family": "Dryden-Peterson",
"given": "Scott",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Dana Farber Cancer Institute , Boston, Massachusetts , USA"
},
{
"name": "Brigham and Women’s Hospital , Boston, Massachusetts , USA"
},
{
"name": "Massachusetts General Hospital , Boston, Massachusetts , USA"
}
],
"family": "Kim",
"given": "Andy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health , Boston, Massachusetts , USA"
},
{
"name": "Brigham and Women’s Hospital , Boston, Massachusetts , USA"
}
],
"family": "Joyce",
"given": "Mary-Ruth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Botswana Harvard AIDS Institute , Gaborone , Botswana"
},
{
"name": "Brigham and Women’s Hospital , Boston, Massachusetts , USA"
},
{
"name": "Harvard Medical School , Boston, Massachusetts , USA"
}
],
"family": "Johnson",
"given": "Jennifer A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Massachusetts General Hospital , Boston, Massachusetts , USA"
}
],
"family": "Kim",
"given": "Arthur Y",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School , Boston, Massachusetts , USA"
},
{
"name": "Brigham and Women’s Hospital , Boston, Massachusetts , USA"
},
{
"name": "Dana Farber Cancer Institute , Boston, Massachusetts , USA"
}
],
"family": "Baden",
"given": "Lindsey R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brigham and Women’s Hospital , Boston, Massachusetts , USA"
},
{
"name": "Dana Farber Cancer Institute , Boston, Massachusetts , USA"
},
{
"name": "Harvard Medical School , Boston, Massachusetts , USA"
}
],
"family": "Woolley",
"given": "Ann E",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
27
]
],
"date-time": "2022-10-27T06:08:00Z",
"timestamp": 1666850880000
},
"deposited": {
"date-parts": [
[
2022,
10,
27
]
],
"date-time": "2022-10-27T06:08:01Z",
"timestamp": 1666850881000
},
"indexed": {
"date-parts": [
[
2022,
10,
27
]
],
"date-time": "2022-10-27T06:42:58Z",
"timestamp": 1666852978424
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
27
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
27
]
],
"date-time": "2022-10-27T00:00:00Z",
"timestamp": 1666828800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac565/46650427/ofac565.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac565/46650427/ofac565.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
10,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
27
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofac565/6775265"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Bebtelovimab for high-risk outpatients with early COVID-19 in a large US health system",
"type": "journal-article"
}