IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections
et al., Frontiers in Medicine, doi:10.3389/fmed.2020.583897, Oct 2020
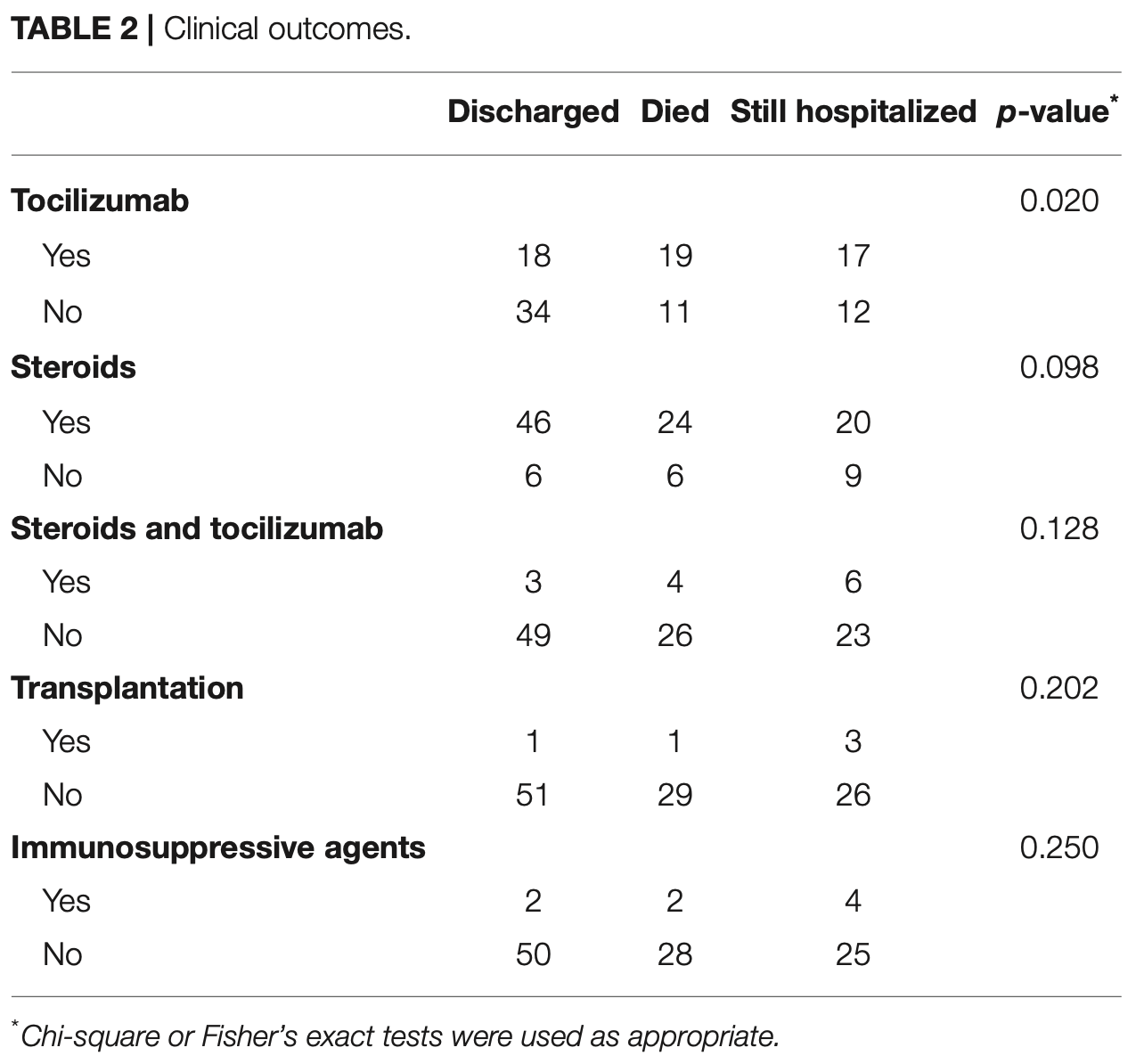
Retrospective 111 critically ill COVID-19 patients showing that tocilizumab treatment was associated with increased secondary bacterial infections, higher mortality (35.2% vs 19.3%, p=0.020), and a trend toward more fungal infections (5.6% vs 0%, p=0.112).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 82.3% higher, RR 1.82, p = 0.09, treatment 19 of 54 (35.2%), control 11 of 57 (19.3%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kimmig et al., 28 Oct 2020, retrospective, USA, peer-reviewed, 9 authors, study period 1 March, 2020 - 27 April, 2020.
Contact: gmutlu@medicine.bsd.uchicago.edu.
IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections
Frontiers in Medicine, doi:10.3389/fmed.2020.583897
Background: Anti-inflammatory therapies such as IL-6 inhibition have been proposed for COVID-19 in a vacuum of evidence-based treatment. However, abrogating the inflammatory response in infectious diseases may impair a desired host response and pre-dispose to secondary infections.
Methods: We retrospectively reviewed the medical record of critically ill COVID-19 patients during an 8-week span and compared the prevalence of secondary infection and outcomes in patients who did and did not receive tocilizumab. Additionally, we included representative histopathologic post-mortem findings from several COVID-19 cases that underwent autopsy at our institution. Results: One hundred eleven patients were identified, of which 54 had received tocilizumab while 57 had not. Receiving tocilizumab was associated with a higher risk of secondary bacterial (48.1 vs. 28.1%; p = 0.029 and fungal (5.6 vs. 0%; p = 0.112) infections. Consistent with higher number of infections, patients who received tocilizumab had higher mortality (35.2 vs. 19.3%; p = 0.020). Seven cases underwent autopsy. In three cases who received tocilizumab, there was evidence of pneumonia on pathology. Of the four cases that had not been given tocilizumab, two showed evidence of aspiration pneumonia and two exhibited diffuse alveolar damage. Conclusions: Experimental therapies are currently being applied to COVID-19 outside of clinical trials. Anti-inflammatory therapies such as anti-IL-6 therapy have the potential to impair viral clearance, pre-dispose to secondary infection, and cause harm. We seek to raise physician awareness of these issues and highlight the need to better understand the immune response in COVID-19.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by University of Chicago IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AUTHOR CONTRIBUTIONS GM, LK, DW, and NP conceived ideas and design. GM, LK, DW, MG, EM, JM, and AH performed data collection. EM, GM, LK, DW, MG, JM, AH, NP, and DP performed data analysis and interpretation. Manuscript drafting and editing was done by GM, LK, DW, EM, NP, and DP. All authors contributed to the article and approved the submitted version.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ali, Kaitha, Mahmood, Ftesi, Stone et al., Clinical use of anti-TNF therapy and increased risk of infections, Drug Healthc Patient Saf, doi:10.2147/DHPS.S28801
Campochiaro, Della-Torre, Cavalli, Luca, Ripa et al., Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study, Eur J Intern Med, doi:10.1016/j.ejim.2020.05.021
Davis, Ferreira, Paige, Gedye, Boyle, Infectious complications of biological and small molecule targeted immunomodulatory therapies, Clin Microbiol Rev, doi:10.1128/CMR.00035-19
Fisher, Jr, Agosti, Opal, Lowry et al., Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The soluble TNF receptor sepsis study group, N Engl J Med, doi:10.1056/NEJM199606273342603
Kimmig, Wu, Gold, Pettit, Pitrak et al., IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections, medRxiv, doi:10.1101/2020.05.15.20103531
Kopf, Baumann, Freer, Freudenberg, Lamers et al., Impaired immune and acute-phase responses in interleukin-6-deficient mice, Nature, doi:10.1038/368339a0
Lang, Englbrecht, Rech, Nusslein, Manger et al., Risk of infections in rheumatoid arthritis patients treated with tocilizumab, Rheumatology, doi:10.1093/rheumatology/ker223
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: a single center experience, J Med Virol, doi:10.1002/jmv.25801
Mansournia, Geroldinger, Greenland, Heinze, Separation in logistic regression: causes, consequences, and control, Am J Epidemiol, doi:10.1093/aje/kwx299
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Morena, Milazzo, Oreni, Bestetti, Fossali et al., Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy, Eur J Intern Med, doi:10.1016/j.ejim.2020.05.011
Narazaki, Kishimoto, The two-faced cytokine il-6 in host defense and diseases, Int J Mol Sci, doi:10.3390/ijms19113528
Pawar, Desai, Solomon, Ortiz, Gale et al., Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study, Ann Rheum Dis, doi:10.1136/annrheumdis-2018-214367
Toniati, Piva, Cattalini, Garrafa, Regola et al., Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy, Autoimmun Rev, doi:10.1016/j.autrev.2020.102568
Velazquez-Salinas, Verdugo-Rodriguez, Rodriguez, Borca, The role of interleukin 6 during viral infections, Front Microbiol, doi:10.3389/fmicb.2019.01057
Xu, Han, Li, Sun, Wang et al., Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci, doi:10.1073/pnas.2005615117
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3389/fmed.2020.583897",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2020.583897",
"alternative-id": [
"10.3389/fmed.2020.583897"
],
"author": [
{
"affiliation": [],
"family": "Kimmig",
"given": "Lucas M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wu",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gold",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pettit",
"given": "Natasha N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pitrak",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mueller",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Husain",
"given": "Aliya N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mutlu",
"given": "Ece A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mutlu",
"given": "Gökhan M.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2020,
10,
28
]
],
"date-time": "2020-10-28T05:45:35Z",
"timestamp": 1603863935000
},
"deposited": {
"date-parts": [
[
2021,
4,
10
]
],
"date-time": "2021-04-10T23:51:13Z",
"timestamp": 1618098673000
},
"funder": [
{
"DOI": "10.13039/100000050",
"award": [
"T32HL007605"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000050",
"id-type": "DOI"
}
],
"name": "National Heart, Lung, and Blood Institute"
},
{
"DOI": "10.13039/100000066",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000066",
"id-type": "DOI"
}
],
"name": "National Institute of Environmental Health Sciences"
},
{
"DOI": "10.13039/100000005",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000005",
"id-type": "DOI"
}
],
"name": "U.S. Department of Defense"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
6
]
],
"date-time": "2025-06-06T08:29:51Z",
"timestamp": 1749198591199,
"version": "3.37.3"
},
"is-referenced-by-count": 110,
"issued": {
"date-parts": [
[
2020,
10,
28
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
28
]
],
"date-time": "2020-10-28T00:00:00Z",
"timestamp": 1603843200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2020.583897/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2020,
10,
28
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
28
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "B1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID-19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"journal-title": "Lancet",
"key": "B2",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/368339a0",
"article-title": "Impaired immune and acute-phase responses in interleukin-6-deficient mice",
"author": "Kopf",
"doi-asserted-by": "publisher",
"first-page": "339",
"journal-title": "Nature",
"key": "B3",
"volume": "368",
"year": "1994"
},
{
"DOI": "10.3390/ijms19113528",
"article-title": "The two-faced cytokine il-6 in host defense and diseases",
"author": "Narazaki",
"doi-asserted-by": "publisher",
"first-page": "3528",
"journal-title": "Int J Mol Sci",
"key": "B4",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1093/rheumatology/ker223",
"article-title": "Risk of infections in rheumatoid arthritis patients treated with tocilizumab",
"author": "Lang",
"doi-asserted-by": "publisher",
"first-page": "852",
"journal-title": "Rheumatology",
"key": "B5",
"volume": "51",
"year": "2012"
},
{
"DOI": "10.1136/annrheumdis-2018-214367",
"article-title": "Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study",
"author": "Pawar",
"doi-asserted-by": "publisher",
"first-page": "456",
"journal-title": "Ann Rheum Dis",
"key": "B6",
"volume": "78",
"year": "2019"
},
{
"DOI": "10.1128/CMR.00035-19",
"article-title": "Infectious complications of biological and small molecule targeted immunomodulatory therapies",
"author": "Davis",
"doi-asserted-by": "crossref",
"key": "B7",
"volume-title": "Clin Microbiol Rev",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2019.01057",
"article-title": "The role of interleukin 6 during viral infections",
"author": "Velazquez-Salinas",
"doi-asserted-by": "publisher",
"first-page": "1057",
"journal-title": "Front Microbiol",
"key": "B8",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1073/pnas.2005615117",
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "10970",
"journal-title": "Proc Natl Acad Sci USA",
"key": "B9",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"article-title": "Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy",
"author": "Toniati",
"doi-asserted-by": "publisher",
"first-page": "102568",
"journal-title": "Autoimmun Rev",
"key": "B10",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.011",
"article-title": "Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy",
"author": "Morena",
"doi-asserted-by": "publisher",
"first-page": "36",
"journal-title": "Eur J Intern Med",
"key": "B11",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25801",
"article-title": "Tocilizumab treatment in COVID-19: a single center experience",
"author": "Luo",
"doi-asserted-by": "publisher",
"first-page": "814",
"journal-title": "J Med Virol",
"key": "B12",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.021",
"article-title": "Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study",
"author": "Campochiaro",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Eur J Intern Med",
"key": "B13",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1093/aje/kwx299",
"article-title": "Separation in logistic regression: causes, consequences, and control",
"author": "Mansournia",
"doi-asserted-by": "publisher",
"first-page": "864",
"journal-title": "Am J Epidemiol",
"key": "B14",
"volume": "187",
"year": "2018"
},
{
"DOI": "10.1056/NEJM199606273342603",
"article-title": "Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The soluble TNF receptor sepsis study group",
"author": "Fisher",
"doi-asserted-by": "publisher",
"first-page": "1697",
"journal-title": "N Engl J Med",
"key": "B15",
"volume": "334",
"year": "1996"
},
{
"DOI": "10.2147/DHPS.S28801",
"article-title": "Clinical use of anti-TNF therapy and increased risk of infections",
"author": "Ali",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Drug Healthc Patient Saf",
"key": "B16",
"volume": "5",
"year": "2013"
},
{
"article-title": "IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections",
"author": "Kimmig",
"key": "B17",
"volume-title": "medRxiv.",
"year": "2020"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.05.15.20103531",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2020.583897/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "7"
}