Histamine-2 Receptor Antagonists and Proton Pump Inhibitors Are Associated With Reduced Risk of SARS-CoV-2 Infection Without Comorbidities Including Diabetes, Hypertension, and Dyslipidemia: A Propensity Score-Matched Nationwide Cohort Study
et al., Journal of Korean Medical Science, doi:10.3346/jkms.2023.38.e99, Mar 2023
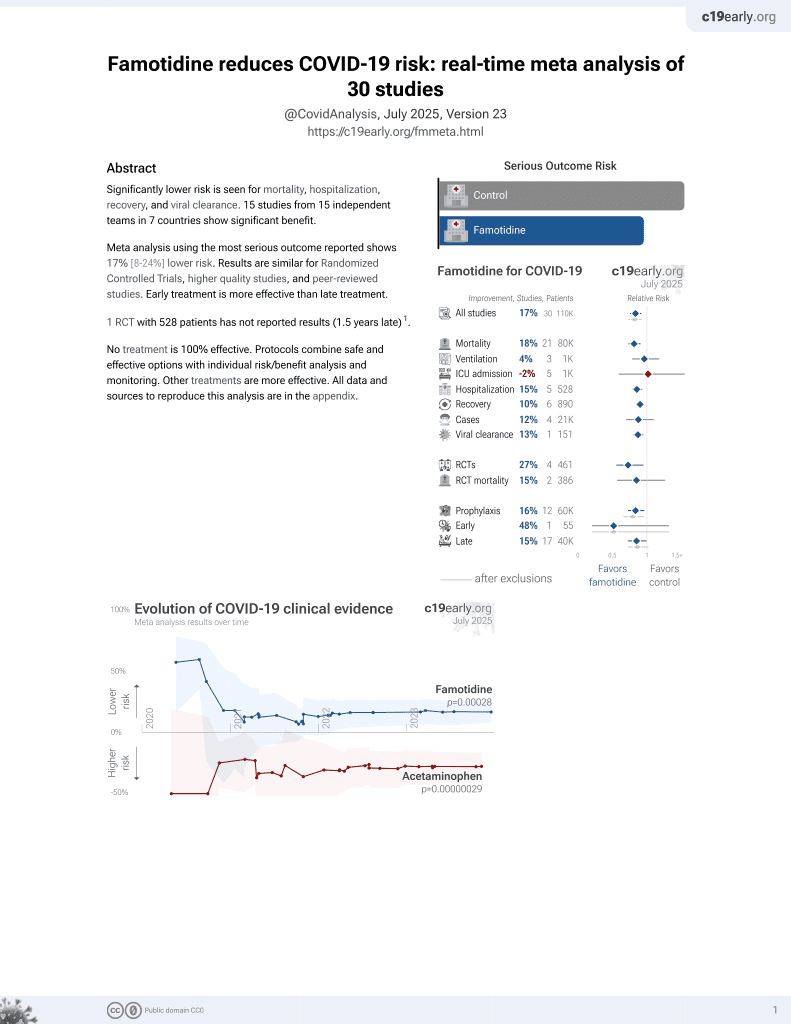
Famotidine for COVID-19
28th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
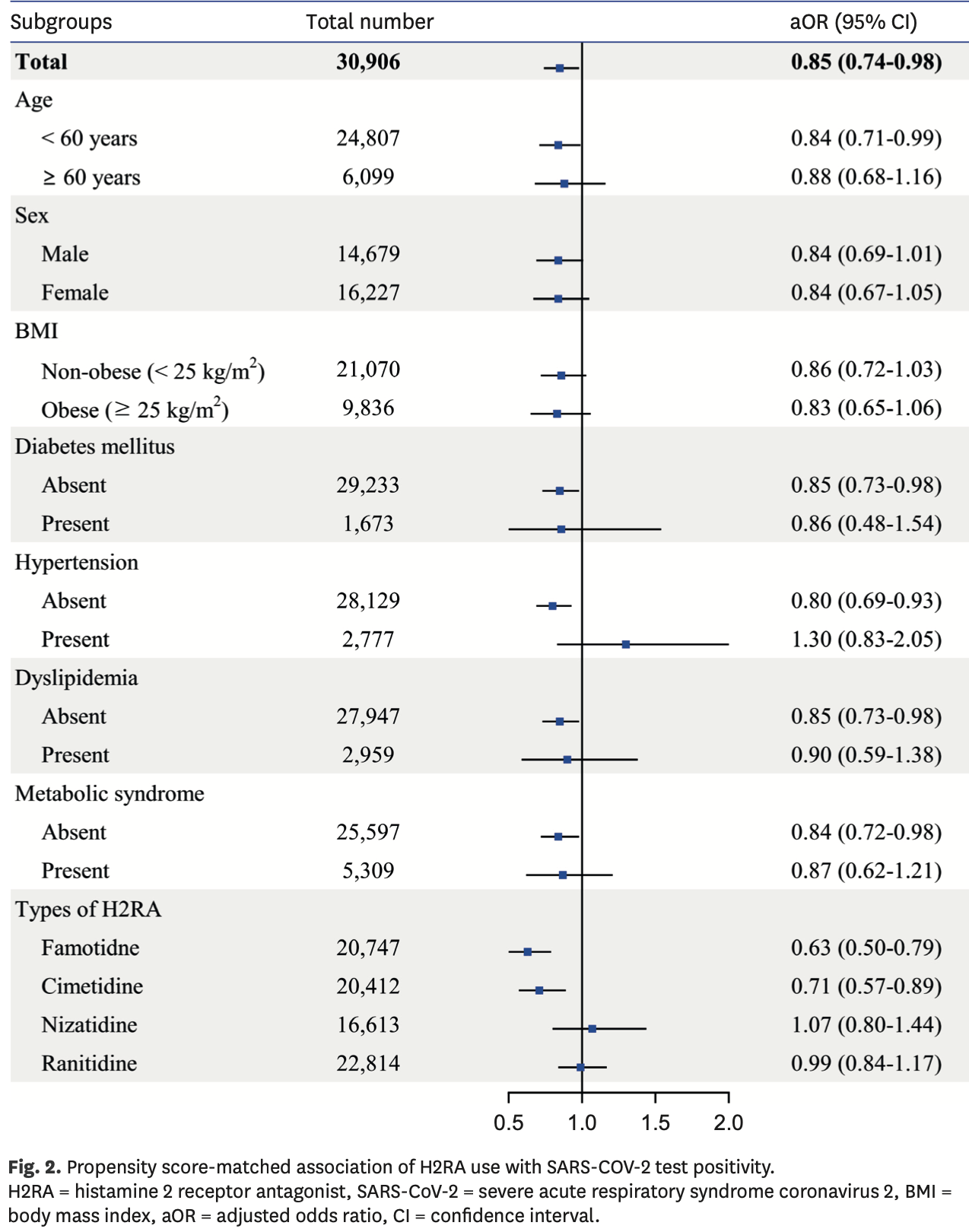
PSM retrospective in South Korea, showing lower risk of COVID-19 cases with H2RA (including famotidine) and PPI use, but no significant difference in severe outcomes (results provided for the combined groups only).
Study covers famotidine and proton pump inhibitors.
|
risk of case, 36.3% lower, RR 0.64, p < 0.001, treatment 105 of 5,594 (1.9%), control 480 of 15,432 (3.1%), NNT 81, adjusted per study, odds ratio converted to relative risk, propensity score matching, multivariable, model 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kim et al., 21 Mar 2023, retrospective, South Korea, peer-reviewed, 8 authors, study period 1 January, 2020 - 4 June, 2020.
Contact: crystal522@daum.net.
Histamine-2 Receptor Antagonists and Proton Pump Inhibitors Are Associated With Reduced Risk of SARS-CoV-2 Infection Without Comorbidities Including Diabetes, Hypertension, and Dyslipidemia: A Propensity Score-Matched Nationwide Cohort Study
Journal of Korean Medical Science, doi:10.3346/jkms.2023.38.e99
Background: This study aimed to identify the effect of histamine-2 receptor antagonist (H2RA) and proton pump inhibitor (PPI) use on the positivity rate and clinical outcomes of coronavirus disease 2019 (COVID-19). Methods: We performed a nationwide cohort study with propensity score matching using medical claims data and general health examination results from the Korean National Health Insurance Service. Individuals aged ≥ 20 years who were tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) between 1 January and 4 June 2020 were included. Patients who were prescribed H2RA or PPI within 1 year of the test date were defined as H2RA and PPI users, respectively. The primary outcome was SARS-CoV-2 test positivity, and the secondary outcome was the instance of severe clinical outcomes of COVID-19, including death, intensive care unit admission, and mechanical ventilation administration. Results: Among 59,094 patients tested for SARS-CoV-2, 21,711 were H2RA users, 12,426 were PPI users, and 24,957 were non-users. After propensity score matching, risk of SARS-CoV-2 infection was significantly lower in H2RA users (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.74-0.98) and PPI users (OR, 0.62; 95% CI, 0.52-0.74) compared to non-users. In patients with comorbidities including diabetes, dyslipidemia, and hypertension, the effect of H2RA and PPI against SARS-CoV-2 infection was not significant, whereas the protective effect was maintained in patients without such comorbidities. Risk of severe clinical outcomes in COVID-19 patients showed no difference between users and non-users after propensity score matching either in H2RA users (OR, 0.89; 95% CI, 0.52-1.54) or PPI users (OR, 1.22; 95% CI, 0.60-2.51). Conclusion: H2RA and PPI use is associated with a decreased risk for SARS-CoV-2 infection but does not affect clinical outcome. Comorbidities including diabetes, hypertension, and dyslipidemia seem to offset the protective effect of H2RA and PPI.
Ethics statement The requirement for written consent from patients was waived by the Institutional Review Board of Seoul National University Hospital (IRB No. 2102-004-1192) based on the observational nature of the study. All patient-related identifiers were anonymized for confidentiality.
SUPPLEMENTARY MATERIALS Supplementary
Supplementary Table 6 Propensity score matched baseline characteristics and SARS-CoV-2 test positivity in H2RA user, PPI user, and non-user groups in the entire cohort (including individuals with and without health examination records) Click here to view Supplementary Table 7 Baseline characteristics of patients diagnosed with COVID-19 according to previous use of H2RA and PPI
Click here to view
Supplementary Table 8 Propensity score matched baseline characteristics and clinical outcomes of COVID-19 patients in H2RA user, PPI user, and non-user groups Click here to view Supplementary Table 9 Comparison of previous studies that evaluated the effect of H2RA and PPI on the risk of SARS-CoV-2 infection and clinical outcomes of COVID-19 Click here to view Supplementary Fig. 1 Flowchart of population selection for clinical outcomes in COVID-19 patients.
Click here to view
References
Alberti, Eckel, Grundy, Zimmet, Cleeman et al., Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity, Circulation
Almario, Chey, Spiegel, Increased risk of COVID-19 among users of proton pump inhibitors, Am J Gastroenterol, doi:10.14309/ajg.0000000000000798
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples, Stat Med, doi:10.1002/sim.3697
Bae, Ghang, Kim, Lim, Yun et al., Recent hydroxychloroquine use is not significantly associated with positive PCR results for SARS-CoV-2: a nationwide observational study in South Korea, Viruses, doi:10.3390/v13020329
Bartoszko, Siemieniuk, Kum, Qasim, Zeraatkar et al., Prophylaxis against COVID-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.n949
Blanc, Waechter, Vogel, Schorr, Demuynck et al., Therapeutic prevention of COVID-19 in elderly: a case-control study, Geroscience
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis, doi:10.1016/0021-9681(87)90171-8
Cho, Choi, Gwon, Metabolic syndrome and the risk of COVID-19 infection: a nationwide population-based case-control study, Nutr Metab Cardiovasc Dis, doi:10.1016/j.numecd.2021.05.016
Dai, Tao, Chen, Tian, Guo et al., Influence of cigarettes and alcohol on the severity and death of COVID-19: a multicenter retrospective study in Wuhan, China, Front Physiol, doi:10.3389/fphys.2020.588553
Darnell, Subbarao, Feinstone, Taylor, Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV, J Virol Methods, doi:10.1016/j.jviromet.2004.06.006
Elmunzer, Wolf, Scheiman, Tierney, Taylor, North American Alliance for the Study of Digestive Manifestations of COVID-19. Association between preadmission acid suppressive medication exposure and severity of illness in patients hospitalized with COVID-19, Gastroenterology, doi:10.1053/j.gastro.2020.11.007
Fan, Liu, Miyata, Dasarathy, Rotroff et al., Effect of acid suppressants on the risk of COVID-19: a propensity score-matched study using UK Biobank, Gastroenterology, doi:10.1053/j.gastro.2020.09.028
Freedberg, Conigliaro, Wang, Tracey, Callahan et al., Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Ghoneim, Butt, Hamid, Shah, Asaad, The incidence of COVID-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: a population-based study, Metabol Open, doi:10.1016/j.metop.2020.100057
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy, JAMA
Guan, Liang, Zhao, Liang, Chen et al., Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J, doi:10.1183/13993003.00547-2020
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Israelsen, Ernst, Lundh, Lundbo, Sandholdt et al., Proton pump inhibitor use is not strongly associated with SARS-CoV-2 related outcomes: a nationwide study and meta-analysis, Clin Gastroenterol Hepatol, doi:10.1016/j.cgh.2021.05.011
Jimenez, Codo, Sampaio, Oliveira, Ferreira et al., Acid pH increases SARS-CoV-2 infection and the risk of death by COVID-19, Front Med, doi:10.3389/fmed.2021.637885
Jung, Choi, You, Kim, Association of renin-angiotensin-aldosterone system inhibitors with coronavirus disease 2019 (COVID-19)-related outcomes in Korea: a nationwide population-based cohort study, Clin Infect Dis, doi:10.1093/cid/ciaa624
Kim, Byeon, Kim, Cho, Lee, The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean National Health Insurance big data, J Korean Med Sci, doi:10.3346/jkms.2020.35.e243
Kim, Kim, Wolf, Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis, Eur J Clin Pharmacol, doi:10.1007/s00228-021-03255-1
Kuswardhani, Henrina, Pranata, Lim, Lawrensia et al., Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis, Diabetes Metab Syndr, doi:10.1016/j.dsx.2020.10.022
Lee, Ha, Yeniova, Moon, Kim et al., Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, Gut, doi:10.1136/gutjnl-2020-322248
Lee, Park, Kim, Han, Kim et al., Appropriate waist circumference cutoff points for central obesity in Korean adults, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2006.04.013
Li, Zhu, Yang, Yan, Xiong et al., Clinical treatment experience in severe and critical COVID-19, Mediators Inflamm, doi:10.1155/2021/9924542
Liu, Sloan, Owings, Figgins, Gauthier et al., Increased ACE2 levels and mortality risk of patients with COVID-19 on proton pump inhibitor therapy, Am J Gastroenterol, doi:10.14309/ajg.0000000000001311
Malone, Tisdall, Smith, Liu, Huang et al., COVID-19: famotidine, histamine, mast cells, and mechanisms, Front Pharmacol, doi:10.3389/fphar.2021.633680
Mauvais-Jarvis, Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19, Diabetes, doi:10.2337/dbi19-0023
Moon, Lee, Park, Yun, Lee et al., Clinical characteristics and mortality predictors of COVID-19 patients hospitalized at nationally-designated treatment hospitals, J Korean Med Sci, doi:10.3346/jkms.2020.35.e328
Muscogiuri, Bettini, Boschetti, Barrea, Savastano et al., Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor?, J Intern Med, doi:10.1111/joim.13121
Oddy, Mccaul, Keeling, Allington, Senn et al., Pharmacological predictors of morbidity and mortality in COVID-19, J Clin Pharmacol, doi:10.1002/jcph.1878
Parsons, Reducing bias in a propensity score matched-pair sample using greedy matching techniques
Pubmed | Crossref, None, doi:10.1007/s11357-021-00397-z
Pubmed | Crossref, None, doi:10.1001/jama.2020.5394
Pubmed | Crossref, None, doi:10.1161/CIRCULATIONAHA.109.192644
Ramachandran, Perisetti, Gajendran, Louis, Bansal et al., Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19, Eur J Gastroenterol Hepatol, doi:10.1097/meg.0000000000002013
Ray, Sharma, Sadasivam, The potential therapeutic role of proton pump inhibitors in COVID-19: hypotheses based on existing evidences, Drug Res (Stuttg), doi:10.1055/a-1236-3041
Sallis, Young, Tartof, Sallis, Sall et al., Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients, Br J Sports Med, doi:10.1136/bjsports-2021-104080
Scalsky, Chen, Desai, Connell, Perry et al., Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK Biobank, PLoS One, doi:10.1371/journal.pone.0248602
Shoaibi, Fortin, Weinstein, Berlin, Ryan, Comparative effectiveness of famotidine in hospitalized COVID-19 patients, Am J Gastroenterol, doi:10.14309/ajg.0000000000001153
Shupp, Mehta, Chirayath, Patel, Aiad et al., Proton pump inhibitor therapy usage and associated hospitalization rates and critical care outcomes of COVID-19 patients, Sci Rep, doi:10.1038/s41598-022-11680-0
Song, Jung, Song, Park, Kwon et al., Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance System, Diabetes Metab J, doi:10.1001/jama.2020.6602
Steenblock, Schwarz, Ludwig, Linkermann, Zimmet et al., COVID-19 and metabolic disease: mechanisms and clinical management, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(21)00244-8
Van Soest, Siersema, Dieleman, Sturkenboom, Kuipers, Persistence and adherence to proton pump inhibitors in daily clinical practice, Aliment Pharmacol Ther, doi:10.1111/j.1365-2036.2006.02982.x
Vila-Corcoles, Satue-Gracia, Ochoa-Gondar, Torrente-Fraga, Gomez-Bertomeu et al., Use of distinct anti-hypertensive drugs and risk for COVID-19 among hypertensive people: a populationbased cohort study in Southern Catalonia, Spain, J Clin Hypertens, doi:10.1111/jch.13948
Wang, Li, Hsieh, Fan, Hsu et al., Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies, Expert Opin Drug Saf, doi:10.1080/14740338.2019.1577820
Who Expert Consultation, Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies, Lancet, doi:10.1016/S0140-6736(03)15268-3
Wu, Liu, Yang, Zhang, Zhong et al., Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharm Sin B, doi:10.1016/j.apsb.2020.02.008
Xiang, Wong, So, Exploring drugs and vaccines associated with altered risks and severity of COVID-19: a UK Biobank cohort study of all ATC level-4 drug categories reveals repositioning opportunities, Pharmaceutics, doi:10.3390/pharmaceutics13091514
Yan, Chen, Sun, Ahmed, Bhan et al., Does proton pump inhibitor use lead to a higher risk of coronavirus disease 2019 infection and progression to severe disease? A meta-analysis, Jpn J Infect Dis, doi:10.7883/yoken.JJID.2021.074
Yanai, Metabolic syndrome and COVID-19, Cardiol Rev, doi:10.14740/cr1181
Yang, George, Thompson, Silverman, Tsaava et al., Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm, Mol Med, doi:10.1186/s10020-022-00483-8
Yao, Ye, Zhang, Cui, Huang et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
Yeramaneni, Doshi, Sands, Cooper, Kurbegov et al., Famotidine use is not associated with 30-day mortality: a coarsened exact match study in 7158 hospitalized patients with coronavirus disease 2019 from a large healthcare system, Gastroenterology, doi:10.1053/j.gastro.2020.10.011
Yozgat, Kasapoğlu, Can, Tanoğlu, Sakin et al., Long-term proton pump inhibitor use is a risk factor for mortality in patients hospitalized for COVID-19, Turk J Med Sci, doi:10.3906/sag-2103-80
Zheng, Kämpfen, Huang, Health-seeking and diagnosis delay and its associated factors: a case study on COVID-19 infections in Shaanxi Province, China, Sci Rep, doi:10.1038/s41598-021-96888-2
Zhou, Wang, Lee, Wu, Cheung et al., Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score-matched territory-wide study, Gut, doi:10.1136/gutjnl-2020-323668
DOI record:
{
"DOI": "10.3346/jkms.2023.38.e99",
"ISSN": [
"1011-8934",
"1598-6357"
],
"URL": "http://dx.doi.org/10.3346/jkms.2023.38.e99",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2022-07-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"value": "2022-12-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published online",
"name": "published_online",
"value": "2023-03-21"
},
{
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Korean Academy of Medical Sciences."
},
{
"explanation": {
"URL": "https://creativecommons.org/licenses/by-nc/4.0/"
},
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "License",
"name": "license",
"value": "This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9143-9654",
"affiliation": [
{
"name": "Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Bokyung",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8920-8777",
"affiliation": [
{
"name": "Department of Biostatistics, The Catholic University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Jung",
"given": "Jin-Hyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9622-0643",
"affiliation": [
{
"name": "Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Han",
"given": "Kyungdo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2332-7420",
"affiliation": [
{
"name": "Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Kang",
"given": "Seungkyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3646-2170",
"affiliation": [
{
"name": "Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Lee",
"given": "Eunwoo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5159-357X",
"affiliation": [
{
"name": "Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Chung",
"given": "Hyunsoo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1799-9028",
"affiliation": [
{
"name": "Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Sang Gyun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7144-0589",
"affiliation": [
{
"name": "Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Cho",
"given": "Soo-Jeong",
"sequence": "additional"
}
],
"container-title": "Journal of Korean Medical Science",
"container-title-short": "J Korean Med Sci",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"jkms.org"
]
},
"created": {
"date-parts": [
[
2023,
3,
22
]
],
"date-time": "2023-03-22T00:07:40Z",
"timestamp": 1679443660000
},
"deposited": {
"date-parts": [
[
2023,
4,
3
]
],
"date-time": "2023-04-03T00:04:48Z",
"timestamp": 1680480288000
},
"funder": [
{
"DOI": "10.13039/501100003725",
"award": [
"NRF-2019R1A2C1009923",
"NRF-2022R1A2B5B01001430"
],
"doi-asserted-by": "publisher",
"name": "National Research Foundation of Korea"
},
{
"DOI": "10.13039/501100017635",
"award": [
"KCHUGR – 202002001"
],
"doi-asserted-by": "publisher",
"name": "Korean College of Helicobacter and Upper Gastrointestinal Research"
},
{
"award": [
"0620211010"
],
"name": "Dong-A Pharmaceutical Co."
}
],
"indexed": {
"date-parts": [
[
2023,
4,
3
]
],
"date-time": "2023-04-03T04:40:38Z",
"timestamp": 1680496838647
},
"is-referenced-by-count": 0,
"issue": "13",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "13",
"published-online": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
}
],
"link": [
{
"URL": "https://jkms.org/pdf/10.3346/jkms.2023.38.e99",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://jkms.org/DOIx.php?id=10.3346/jkms.2023.38.e99",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://jkms.org/DOIx.php?id=10.3346/jkms.2023.38.e99",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "18617",
"original-title": [],
"prefix": "10.3346",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"publisher": "XMLink",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.3346/jkms.2023.38.e99_ref1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1574",
"issue": "16",
"journal-title": "JAMA",
"key": "10.3346/jkms.2023.38.e99_ref2",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.2337/dbi19-0023",
"author": "Mauvais-Jarvis",
"doi-asserted-by": "crossref",
"first-page": "1857",
"issue": "9",
"journal-title": "Diabetes",
"key": "10.3346/jkms.2023.38.e99_ref3",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00547-2020",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "2000547",
"issue": "5",
"journal-title": "Eur Respir J",
"key": "10.3346/jkms.2023.38.e99_ref4",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2020.35.e328",
"author": "Moon",
"doi-asserted-by": "crossref",
"first-page": "e328",
"issue": "36",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2023.38.e99_ref5",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2020.35.e243",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "e243",
"issue": "26",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2023.38.e99_ref6",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1038/s41598-022-11680-0",
"author": "Shupp",
"doi-asserted-by": "crossref",
"first-page": "7596",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.3346/jkms.2023.38.e99_ref7",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "766",
"issue": "5",
"journal-title": "Acta Pharm Sin B",
"key": "10.3346/jkms.2023.38.e99_ref8",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.jviromet.2004.06.006",
"author": "Darnell",
"doi-asserted-by": "crossref",
"first-page": "85",
"issue": "1",
"journal-title": "J Virol Methods",
"key": "10.3346/jkms.2023.38.e99_ref9",
"volume": "121",
"year": "2004"
},
{
"DOI": "10.3389/fmed.2021.637885",
"author": "Jimenez",
"doi-asserted-by": "crossref",
"first-page": "637885",
"journal-title": "Front Med (Lausanne)",
"key": "10.3346/jkms.2023.38.e99_ref10",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.14309/ajg.0000000000000798",
"author": "Almario",
"doi-asserted-by": "crossref",
"first-page": "1707",
"issue": "10",
"journal-title": "Am J Gastroenterol",
"key": "10.3346/jkms.2023.38.e99_ref11",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-322248",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "76",
"issue": "1",
"journal-title": "Gut",
"key": "10.3346/jkms.2023.38.e99_ref12",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1053/j.gastro.2020.09.028",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "455",
"issue": "1",
"journal-title": "Gastroenterology",
"key": "10.3346/jkms.2023.38.e99_ref13",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1007/s11357-021-00397-z",
"author": "Blanc",
"doi-asserted-by": "crossref",
"first-page": "2333",
"issue": "5",
"journal-title": "Geroscience",
"key": "10.3346/jkms.2023.38.e99_ref14",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1007/s00228-021-03255-1",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "383",
"issue": "3",
"journal-title": "Eur J Clin Pharmacol",
"key": "10.3346/jkms.2023.38.e99_ref15",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.1111/jch.13948",
"author": "Vila-Corcoles",
"doi-asserted-by": "crossref",
"first-page": "1379",
"issue": "8",
"journal-title": "J Clin Hypertens (Greenwich)",
"key": "10.3346/jkms.2023.38.e99_ref16",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.14309/ajg.0000000000001153",
"author": "Shoaibi",
"doi-asserted-by": "crossref",
"first-page": "692",
"issue": "4",
"journal-title": "Am J Gastroenterol",
"key": "10.3346/jkms.2023.38.e99_ref17",
"volume": "116",
"year": "2021"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"author": "Freedberg",
"doi-asserted-by": "crossref",
"first-page": "1129",
"issue": "3",
"journal-title": "Gastroenterology",
"key": "10.3346/jkms.2023.38.e99_ref18",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.10.011",
"author": "Yeramaneni",
"doi-asserted-by": "crossref",
"first-page": "919",
"issue": "3",
"journal-title": "Gastroenterology",
"key": "10.3346/jkms.2023.38.e99_ref19",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1097/MEG.0000000000002013",
"author": "Ramachandran",
"doi-asserted-by": "crossref",
"first-page": "137",
"issue": "2",
"journal-title": "Eur J Gastroenterol Hepatol",
"key": "10.3346/jkms.2023.38.e99_ref20",
"volume": "34",
"year": "2022"
},
{
"DOI": "10.1053/j.gastro.2020.11.007",
"author": "Elmunzer",
"doi-asserted-by": "crossref",
"first-page": "1417",
"issue": "4",
"journal-title": "Gastroenterology",
"key": "10.3346/jkms.2023.38.e99_ref21",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2020-323668",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "2012",
"issue": "10",
"journal-title": "Gut",
"key": "10.3346/jkms.2023.38.e99_ref22",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.4093/dmj.2014.38.5.395",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "395",
"issue": "5",
"journal-title": "Diabetes Metab J",
"key": "10.3346/jkms.2023.38.e99_ref23",
"volume": "38",
"year": "2014"
},
{
"DOI": "10.1001/jama.2020.6602",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "2129",
"issue": "21",
"journal-title": "JAMA",
"key": "10.3346/jkms.2023.38.e99_ref24",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.3390/v13020329",
"author": "Bae",
"doi-asserted-by": "crossref",
"first-page": "329",
"issue": "2",
"journal-title": "Viruses",
"key": "10.3346/jkms.2023.38.e99_ref25",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1080/14740338.2019.1577820",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "163",
"issue": "3",
"journal-title": "Expert Opin Drug Saf",
"key": "10.3346/jkms.2023.38.e99_ref26",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "373",
"issue": "5",
"journal-title": "J Chronic Dis",
"key": "10.3346/jkms.2023.38.e99_ref27",
"volume": "40",
"year": "1987"
},
{
"DOI": "10.1016/j.metop.2020.100057",
"author": "Ghoneim",
"doi-asserted-by": "crossref",
"first-page": "100057",
"journal-title": "Metabol Open",
"key": "10.3346/jkms.2023.38.e99_ref28",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0248602",
"author": "Scalsky",
"doi-asserted-by": "crossref",
"first-page": "e0248602",
"issue": "4",
"journal-title": "PLoS One",
"key": "10.3346/jkms.2023.38.e99_ref29",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1136/bjsports-2021-104080",
"author": "Sallis",
"doi-asserted-by": "crossref",
"first-page": "1099",
"issue": "19",
"journal-title": "Br J Sports Med",
"key": "10.3346/jkms.2023.38.e99_ref30",
"volume": "55",
"year": "2021"
},
{
"DOI": "10.3389/fphys.2020.588553",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "588553",
"journal-title": "Front Physiol",
"key": "10.3346/jkms.2023.38.e99_ref31",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(03)15268-3",
"author": "WHO Expert Consultation",
"doi-asserted-by": "crossref",
"first-page": "157",
"issue": "9403",
"journal-title": "Lancet",
"key": "10.3346/jkms.2023.38.e99_ref32",
"volume": "363",
"year": "2004"
},
{
"DOI": "10.1161/CIRCULATIONAHA.109.192644",
"author": "Alberti",
"doi-asserted-by": "crossref",
"first-page": "1640",
"issue": "16",
"journal-title": "Circulation",
"key": "10.3346/jkms.2023.38.e99_ref33",
"volume": "120",
"year": "2009"
},
{
"DOI": "10.1016/j.diabres.2006.04.013",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "72",
"issue": "1",
"journal-title": "Diabetes Res Clin Pract",
"key": "10.3346/jkms.2023.38.e99_ref34",
"volume": "75",
"year": "2007"
},
{
"DOI": "10.1093/cid/ciaa624",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "2121",
"issue": "16",
"journal-title": "Clin Infect Dis",
"key": "10.3346/jkms.2023.38.e99_ref35",
"volume": "71",
"year": "2020"
},
{
"author": "Parsons",
"first-page": "214",
"key": "10.3346/jkms.2023.38.e99_ref36",
"volume-title": "Reducing bias in a propensity score matched-pair sample using greedy matching techniques",
"year": "2001"
},
{
"DOI": "10.1016/j.dsx.2020.10.022",
"author": "Tuty Kuswardhani",
"doi-asserted-by": "crossref",
"first-page": "2103",
"issue": "6",
"journal-title": "Diabetes Metab Syndr",
"key": "10.3346/jkms.2023.38.e99_ref37",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1002/sim.3697",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "3083",
"issue": "25",
"journal-title": "Stat Med",
"key": "10.3346/jkms.2023.38.e99_ref38",
"volume": "28",
"year": "2009"
},
{
"DOI": "10.1016/j.numecd.2021.05.016",
"author": "Cho",
"doi-asserted-by": "crossref",
"first-page": "2596",
"issue": "9",
"journal-title": "Nutr Metab Cardiovasc Dis",
"key": "10.3346/jkms.2023.38.e99_ref39",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1038/s41366-022-01111-5",
"author": "Muscogiuri",
"doi-asserted-by": "crossref",
"first-page": "1254",
"issue": "7",
"journal-title": "Int J Obes",
"key": "10.3346/jkms.2023.38.e99_ref40",
"volume": "46",
"year": "2022"
},
{
"DOI": "10.1111/joim.13121",
"author": "Luxenburger",
"doi-asserted-by": "crossref",
"first-page": "121",
"issue": "1",
"journal-title": "J Intern Med",
"key": "10.3346/jkms.2023.38.e99_ref41",
"volume": "289",
"year": "2021"
},
{
"DOI": "10.1016/j.cgh.2021.05.011",
"author": "Israelsen",
"doi-asserted-by": "crossref",
"first-page": "1845",
"issue": "9",
"journal-title": "Clin Gastroenterol Hepatol",
"key": "10.3346/jkms.2023.38.e99_ref42",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.14309/ajg.0000000000001311",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1638",
"issue": "8",
"journal-title": "Am J Gastroenterol",
"key": "10.3346/jkms.2023.38.e99_ref43",
"volume": "116",
"year": "2021"
},
{
"DOI": "10.1002/jcph.1878",
"author": "Oddy",
"doi-asserted-by": "crossref",
"first-page": "1286",
"issue": "10",
"journal-title": "J Clin Pharmacol",
"key": "10.3346/jkms.2023.38.e99_ref44",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.3906/sag-2103-80",
"author": "Yozgat",
"doi-asserted-by": "crossref",
"first-page": "1675",
"issue": "3",
"journal-title": "Turk J Med Sci",
"key": "10.3346/jkms.2023.38.e99_ref45",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.7883/yoken.JJID.2021.074",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "10",
"issue": "1",
"journal-title": "Jpn J Infect Dis",
"key": "10.3346/jkms.2023.38.e99_ref46",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.633680",
"author": "Malone",
"doi-asserted-by": "crossref",
"first-page": "633680",
"journal-title": "Front Pharmacol",
"key": "10.3346/jkms.2023.38.e99_ref47",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1055/a-1236-3041",
"author": "Ray",
"doi-asserted-by": "crossref",
"first-page": "484",
"issue": "10",
"journal-title": "Drug Res (Stuttg)",
"key": "10.3346/jkms.2023.38.e99_ref48",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1136/bmj.n949",
"author": "Bartoszko",
"doi-asserted-by": "crossref",
"first-page": "n949",
"issue": "949",
"journal-title": "BMJ",
"key": "10.3346/jkms.2023.38.e99_ref49",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa237",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"issue": "15",
"journal-title": "Clin Infect Dis",
"key": "10.3346/jkms.2023.38.e99_ref50",
"volume": "71",
"year": "2020"
},
{
"author": "Yanai",
"first-page": "360",
"issue": "6",
"journal-title": "Cardiol Rev",
"key": "10.3346/jkms.2023.38.e99_ref51",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(21)00244-8",
"author": "Steenblock",
"doi-asserted-by": "crossref",
"first-page": "786",
"issue": "11",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "10.3346/jkms.2023.38.e99_ref52",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/s10020-022-00483-8",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "57",
"issue": "1",
"journal-title": "Mol Med",
"key": "10.3346/jkms.2023.38.e99_ref53",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-96888-2",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "17331",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.3346/jkms.2023.38.e99_ref54",
"volume": "11",
"year": "2021"
},
{
"author": "Li",
"first-page": "9924542",
"journal-title": "Mediators Inflamm",
"key": "10.3346/jkms.2023.38.e99_ref55",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1111/j.1365-2036.2006.02982.x",
"author": "Van Soest",
"doi-asserted-by": "crossref",
"first-page": "377",
"issue": "2",
"journal-title": "Aliment Pharmacol Ther",
"key": "10.3346/jkms.2023.38.e99_ref56",
"volume": "24",
"year": "2006"
},
{
"DOI": "10.3390/pharmaceutics13091514",
"author": "Xiang",
"doi-asserted-by": "crossref",
"first-page": "1514",
"issue": "9",
"journal-title": "Pharmaceutics",
"key": "10.3346/jkms.2023.38.e99_ref57",
"volume": "13",
"year": "2021"
}
],
"reference-count": 57,
"references-count": 57,
"relation": {},
"resource": {
"primary": {
"URL": "https://jkms.org/DOIx.php?id=10.3346/jkms.2023.38.e99"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Histamine-2 Receptor Antagonists and Proton Pump Inhibitors Are Associated With Reduced Risk of SARS-CoV-2 Infection Without Comorbidities Including Diabetes, Hypertension, and Dyslipidemia: A Propensity Score-Matched Nationwide Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3346/crossmark_policy",
"volume": "38"
}