Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults
et al., Nature Medicine, doi:10.1038/s41591-022-01780-9, NCT04865237, Mar 2022
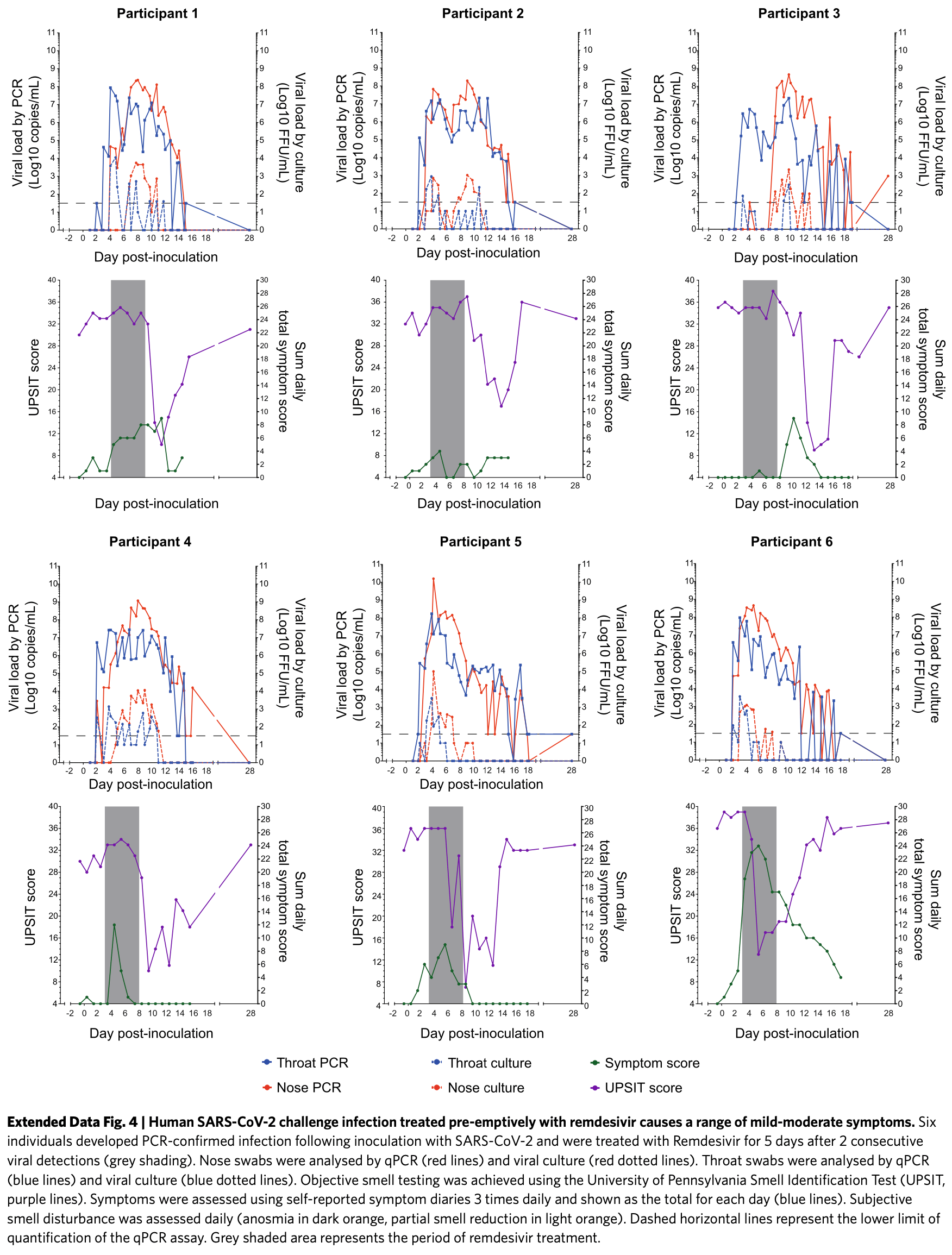
SARS-CoV-2 challenge study in 36 low-risk young adults. Infected participants had high viral loads peaking around 5 days post-exposure, mild-to-moderate upper respiratory symptoms, and anosmia, but no severe disease. Remdesivir had no significant effect on viral kinetics or symptoms. There was a 1-2 day delay before significant viral spread. A majority of patients reported symptoms prior to significant viral spread, supporting the use of early treatment targeted at the upper respiratory tract as a promising approach to limit progression of SARS-CoV-2.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments15.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
peak symptom score, 60.1% higher, RR 1.60, p = 0.43, treatment mean 8.48 (±8.1) n=6, control mean 5.3 (±7.7) n=12, relative peak symptom score.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Killingley et al., 31 Mar 2022, prospective, United Kingdom, peer-reviewed, mean age 21.8, 31 authors, study period March 2021 - July 2021, trial NCT04865237 (history).
Contact: c.chiu@imperial.ac.uk.
Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults
Nature Medicine, doi:10.1038/s41591-022-01780-9
oronavirus disease 2019 (COVID-19) is a complex clinical syndrome caused by SARS-CoV-2. Despite extensive research into severe disease of hospitalized patients 1 and many large studies leading to approval of vaccines and antivirals 2-4 , the global spread of SARS-CoV-2 continues and is, indeed, accelerating in many regions. Infections are typically mild or asymptomatic in younger people, but these likely drive community transmission 5 , and the detailed time course of infection and infectivity in this context has not been fully elucidated 6, 7 . Deliberate human infection of low-risk volunteers enables the exact longitudinal measurement of viral kinetics, immunological responses, transmission dynamics and duration of infectious shedding after a fixed dose of
Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults
Online content Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/ s41591-022-01780-9 .
Articles
Nature MediciNe V-Spot image analyzer, with virus titer determined by calculating the average spot number and subtraction of background spot count from the negative control wells. Serum antibody assays. Serum samples were analyzed at Nexelis to determine SARS-CoV-2 anti-spike IgG concentrations by ELISA (reported as ELU ml -1 ). Neutralizing antibody titers for live SARS-CoV-2 virus (lineage Victoria/01/2020) were determined by microneutralization assay at the UK Health Security Agency and reported as the 50% neutralizing antibody titer (NT 50 ). For the microneutralization assay, lower limit of detection (LLOD) was 58, and undetectable samples were assigned a value of 29. For the spike protein IgG ELISA, LLOD was 50.2 ELU ml -1 , and undetectable samples were assigned a value of 25 ELU ml -1 . Lateral flow rapid antigen assays. LFAs were performed using the Innova SARS-CoV-2 antigen rapid quantitative test kit (BT1309) as per the manufacturer's recommendations with adaptations as follows. This commercially available kit is designed to detect the presence of the SARS-CoV-2 nucleocapsid protein through in vitro..
References
Abdelnabi, Comparing infectivity and virulence of emerging SARS-CoV-2 variants in Syrian hamsters, EBioMedicine
Baay, Neels, SARS-CoV-2 controlled human infection models: ethics, challenge agent production and regulatory issues, Biologicals
Bagga, Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals, Antivir. Ther
Baggio, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in the upper respiratory tract of children and adults with early acute coronavirus disease 2019 (COVID-19), Clin. Infect. Dis
Barker, Public attitudes to a human challenge study with SARS-CoV-2: a mixed-methods study, doi:10.12688/wellcomeopenres.17516.1
Barratt-Due, Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomized trial, Ann. Intern. Med
Beigel, Remdesivir for the treatment of Covid-19-final report
Butowt, Von Bartheld, Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection, Neuroscientist
Chiu, Chiu, /or interpreted the data
Clift, Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study, BMJ
Corbett, Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates, N. Engl. J. Med
Deming, Michael, Robb, Cohen, Neuzil, Accelerating development of SARS-CoV-2 vaccines-the role for controlled human infection models, N. Engl. J. Med
Devincenzo, Viral load drives disease in humans experimentally infected with respiratory syncytial virus, Am. J. Respir. Crit. Care Med
Doty, Frye, Agrawal, Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test, Percept. Psychophys
Drake, Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study, Lancet
Eberhardt, RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients, Viruses
Fajnzylber, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat. Commun
Fischerii, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci. Transl. Med
Gbesemete, Exploring the acceptability of controlled human infection with SARSCoV2-a public consultation, BMC Med
Gottlieb, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N. Engl. J. Med
Habibi, Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection, Science
He, Guo, Mao, Zhang, Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis, J. Med. Virol
He, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat. Med
Hou, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Imai, Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development, Proc. Natl Acad. Sci. USA
Jozwik, RSV-specific airway resident memory CD8 + T cells and differential disease severity after experimental human infection, Nat. Commun
Ke, Zitzmann, Ho, Ribeiro, Perelson, In vivo kinetics of SARS-CoV-2 infection and its relationship with a person's infectiousness, Proc. Natl Acad. Sci. USA
Kim, Duration of culturable SARS-CoV-2 in hospitalized patients with Covid-19, N. Engl. J. Med
Lauer, The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann. Intern. Med
Levine, Viewpoint of a WHO advisory group tasked to consider establishing a closely-monitored challenge model of coronavirus disease 2019 (COVID-19) in healthy volunteers, Clin. Infect. Dis
Levine-Tiefenbrun, Viral loads of delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2, Nat. Med
Liang, Zeger, Longitudinal data analysis using generalized linear models, Biometrika
Marks, Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study, Lancet Infect. Dis
Munker, Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients, Eur. Respir. J
Nguyen-Van-Tam, Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment, PLoS Pathog
Paterson, Innate-like gene expression of lung-resident memory CD8, Am. J. Respir. Crit. Care Med
Petersen, Crozier, Buchan, Mina, Bartlett, Recalibrating SARS-CoV-2 antigen rapid lateral flow test relative sensitivity from validation studies to absolute sensitivity for indicating individuals shedding transmissible virus, Clin. Epidemiol
Quilty, Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study, Lancet Public Health
Rapeport, SARS-CoV-2 human challenge studies-establishing the model during an evolving pandemic, N. Engl. J. Med
Reed, The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: evidence of heterogeneity among 229E-related strains, J. Med. Virol
Renaud, Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis, JAMA Netw. Open
Rosenau, Experiments to determine mode of spread of influenza, JAMA
Rosenke, Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection, Emerg. Microbes Infect
Sia, Pathogenesis and transmission of SARS-CoV-2 in golden hamsters, Nature
Singanayagam, Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study, Lancet Infect. Dis
Syangtan, Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis, Front. Public Health
Thomas, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 Months, N. Engl. J. Med
Voysey, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Walsh, SARS-CoV-2 detection, viral load and infectivity over the course of an infection, J. Infect
Williamson, Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2, Nature
Wölfel, Virological assessment of hospitalized patients with COVID-2019, Nature
Yu, Zhu, Zhang, Han, A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period, J. Infect. Dis
Zar, Biostatistical Analysis 5th edn
Zou, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N. Engl. J. Med
DOI record:
{
"DOI": "10.1038/s41591-022-01780-9",
"ISSN": [
"1078-8956",
"1546-170X"
],
"URL": "http://dx.doi.org/10.1038/s41591-022-01780-9",
"alternative-id": [
"1780"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 November 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "9 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "31 March 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "A.B., A.P.C., A.M., B.Z.L., M.K. and N.N. are employees of hVIVO Services Ltd. and hold shares in Open Orphan and Poolbeg Pharma. A.G. is previous employee at hVIVO Services Ltd. and holds shares in Open Orphan and Poolbeg Pharma. G.R. is a co-founder of SubIntro Ltd. M.G.S. is Director of MedEx Solutions Ltd., chairs the Infectious Disease Scientific Advisory Board of Integrum Scientific LLC and holds shares in Integrum Scientific LLC and MedEx Solutions Ltd. T.W. has received research grants and/or fees from AstraZeneca, UCB, Bergenbio, Synairgen, Valneva and my mhealth (MMH) in the field of COVID-19 research. T.W. owns shares in Open Orphan and MMH. T.W. is a director of MMH. J.S.N.-V.-T. is seconded to the Department of Health and Social Care, England."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"affiliation": [],
"family": "Killingley",
"given": "Ben",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3744-4604",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mann",
"given": "Alex J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalinova",
"given": "Mariya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyers",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goonawardane",
"given": "Niluka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Jie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindsell",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hare",
"given": "Samanjit S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6849-3962",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brown",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frise",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hopkins",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noulin",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Löndt",
"given": "Brandon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkinson",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harden",
"given": "Stephen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2126-5142",
"affiliation": [],
"authenticated-orcid": false,
"family": "McShane",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baillet",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilbert",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobs",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charman",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mande",
"given": "Priya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen-Van-Tam",
"given": "Jonathan S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9700-0418",
"affiliation": [],
"authenticated-orcid": false,
"family": "Semple",
"given": "Malcolm G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4297-6728",
"affiliation": [],
"authenticated-orcid": false,
"family": "Read",
"given": "Robert C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1154-8093",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ferguson",
"given": "Neil M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7220-2555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Openshaw",
"given": "Peter J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5795-6500",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rapeport",
"given": "Garth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3948-0895",
"affiliation": [],
"authenticated-orcid": false,
"family": "Barclay",
"given": "Wendy S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Catchpole",
"given": "Andrew P.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0914-920X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chiu",
"given": "Christopher",
"sequence": "additional"
}
],
"container-title": "Nature Medicine",
"container-title-short": "Nat Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
31
]
],
"date-time": "2022-03-31T06:04:10Z",
"timestamp": 1648706650000
},
"deposited": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T16:05:44Z",
"timestamp": 1652889944000
},
"funder": [
{
"name": "Her Majesty’s Government UK through the Department of Business Energy and Industrial Strategy"
}
],
"indexed": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T18:36:10Z",
"timestamp": 1732041370617
},
"is-referenced-by-count": 324,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
3,
31
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2022,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
31
]
],
"date-time": "2022-03-31T00:00:00Z",
"timestamp": 1648684800000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
31
]
],
"date-time": "2022-03-31T00:00:00Z",
"timestamp": 1648684800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41591-022-01780-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41591-022-01780-9",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41591-022-01780-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "1031-1041",
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
3,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
31
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0140-6736(21)00799-6",
"author": "TM Drake",
"doi-asserted-by": "publisher",
"first-page": "223",
"journal-title": "Lancet",
"key": "1780_CR1",
"unstructured": "Drake, T. M. et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet 398, 223–237 (2021).",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"author": "M Voysey",
"doi-asserted-by": "publisher",
"first-page": "99",
"journal-title": "Lancet",
"key": "1780_CR2",
"unstructured": "Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2110345",
"author": "SJ Thomas",
"doi-asserted-by": "publisher",
"first-page": "1761",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR3",
"unstructured": "Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 Months. N. Engl. J. Med. 385, 1761–1773 (2021).",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"author": "WA Fischerii",
"doi-asserted-by": "publisher",
"first-page": "eabl7430",
"journal-title": "Sci. Transl. Med.",
"key": "1780_CR4",
"unstructured": "Fischerii, W. A. et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 14, eabl7430 (2022).",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/jmv.26326",
"author": "J He",
"doi-asserted-by": "publisher",
"first-page": "820",
"journal-title": "J. Med. Virol.",
"key": "1780_CR5",
"unstructured": "He, J., Guo, Y., Mao, R. & Zhang, J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J. Med. Virol. 93, 820–830 (2021).",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"author": "X He",
"doi-asserted-by": "publisher",
"first-page": "672",
"journal-title": "Nat. Med.",
"key": "1780_CR6",
"unstructured": "He, X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2020.587374",
"author": "G Syangtan",
"doi-asserted-by": "publisher",
"first-page": "587374",
"journal-title": "Front. Public Health",
"key": "1780_CR7",
"unstructured": "Syangtan, G. et al. Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis. Front. Public Health 8, 587374 (2020).",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/ncomms10224",
"author": "A Jozwik",
"doi-asserted-by": "publisher",
"journal-title": "Nat. Commun.",
"key": "1780_CR8",
"unstructured": "Jozwik, A. et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 6, 10224 (2015).",
"volume": "6",
"year": "2015"
},
{
"DOI": "10.1164/rccm.202103-0620OC",
"author": "S Paterson",
"doi-asserted-by": "publisher",
"first-page": "826",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "1780_CR9",
"unstructured": "Paterson, S. et al. Innate-like gene expression of lung-resident memory CD8. Am. J. Respir. Crit. Care Med. 204, 826–841 (2021).",
"volume": "204",
"year": "2021"
},
{
"DOI": "10.1002/jmv.1890130208",
"author": "SE Reed",
"doi-asserted-by": "publisher",
"first-page": "179",
"journal-title": "J. Med. Virol.",
"key": "1780_CR10",
"unstructured": "Reed, S. E. The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: evidence of heterogeneity among 229E-related strains. J. Med. Virol. 13, 179–192 (1984).",
"volume": "13",
"year": "1984"
},
{
"DOI": "10.1126/science.aba9301",
"author": "MS Habibi",
"doi-asserted-by": "publisher",
"first-page": "eaba9301",
"journal-title": "Science",
"key": "1780_CR11",
"unstructured": "Habibi, M. S. et al. Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science 370, eaba9301 (2020).",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1164/rccm.201002-0221OC",
"author": "JP DeVincenzo",
"doi-asserted-by": "publisher",
"first-page": "1305",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "1780_CR12",
"unstructured": "DeVincenzo, J. P. et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 182, 1305–1314 (2010).",
"volume": "182",
"year": "2010"
},
{
"DOI": "10.3851/IMP2629",
"author": "B Bagga",
"doi-asserted-by": "publisher",
"first-page": "785",
"journal-title": "Antivir. Ther.",
"key": "1780_CR13",
"unstructured": "Bagga, B. et al. Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals. Antivir. Ther. 18, 785–791 (2013).",
"volume": "18",
"year": "2013"
},
{
"DOI": "10.1371/journal.ppat.1008704",
"author": "JS Nguyen-Van-Tam",
"doi-asserted-by": "publisher",
"first-page": "e1008704",
"journal-title": "PLoS Pathog.",
"key": "1780_CR14",
"unstructured": "Nguyen-Van-Tam, J. S. et al. Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment. PLoS Pathog. 16, e1008704 (2020).",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1001/jama.1919.02610310005002",
"author": "M Rosenau",
"doi-asserted-by": "publisher",
"first-page": "311",
"journal-title": "JAMA",
"key": "1780_CR15",
"unstructured": "Rosenau, M. Experiments to determine mode of spread of influenza. JAMA 73, 311–313 (1919).",
"volume": "73",
"year": "1919"
},
{
"DOI": "10.1056/NEJMp2106970",
"author": "G Rapeport",
"doi-asserted-by": "publisher",
"first-page": "961",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR16",
"unstructured": "Rapeport, G. et al. SARS-CoV-2 human challenge studies—establishing the model during an evolving pandemic. N. Engl. J. Med. 385, 961–964 (2021).",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1290",
"author": "MM Levine",
"doi-asserted-by": "publisher",
"first-page": "2035",
"journal-title": "Clin. Infect. Dis.",
"key": "1780_CR17",
"unstructured": "Levine, M. M. et al. Viewpoint of a WHO advisory group tasked to consider establishing a closely-monitored challenge model of coronavirus disease 2019 (COVID-19) in healthy volunteers. Clin. Infect. Dis. 72, 2035–2041 (2021).",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1056/NEJMp2020076",
"author": "ME Deming",
"doi-asserted-by": "publisher",
"first-page": "e63",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR18",
"unstructured": "Deming, M. E., Michael, N. L., Robb, M., Cohen, M. S. & Neuzil, K. M. Accelerating development of SARS-CoV-2 vaccines—the role for controlled human infection models. N. Engl. J. Med. 383, e63 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(21)00648-4",
"author": "A Singanayagam",
"doi-asserted-by": "publisher",
"first-page": "183",
"journal-title": "Lancet Infect. Dis.",
"key": "1780_CR19",
"unstructured": "Singanayagam, A. et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect. Dis. 22, 183–195 (2021).",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.biologicals.2020.08.006",
"author": "M Baay",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "Biologicals",
"key": "1780_CR20",
"unstructured": "Baay, M. & Neels, P. SARS-CoV-2 controlled human infection models: ethics, challenge agent production and regulatory issues. Biologicals 67, 69–74 (2020).",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.15352",
"author": "M Renaud",
"doi-asserted-by": "publisher",
"first-page": "e2115352",
"journal-title": "JAMA Netw. Open",
"key": "1780_CR21",
"unstructured": "Renaud, M. et al. Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis. JAMA Netw. Open 4, e2115352 (2021).",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2009799117",
"author": "M Imai",
"doi-asserted-by": "publisher",
"first-page": "16587",
"journal-title": "Proc. Natl Acad. Sci. USA",
"key": "1780_CR22",
"unstructured": "Imai, M. et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl Acad. Sci. USA 117, 16587–16595 (2020).",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2342-5",
"author": "SF Sia",
"doi-asserted-by": "publisher",
"first-page": "834",
"journal-title": "Nature",
"key": "1780_CR23",
"unstructured": "Sia, S. F. et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583, 834–838 (2020).",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2021.103403",
"author": "R Abdelnabi",
"doi-asserted-by": "publisher",
"first-page": "103403",
"journal-title": "EBioMedicine",
"key": "1780_CR24",
"unstructured": "Abdelnabi, R. et al. Comparing infectivity and virulence of emerging SARS-CoV-2 variants in Syrian hamsters. EBioMedicine 68, 103403 (2021).",
"volume": "68",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"author": "YJ Hou",
"doi-asserted-by": "publisher",
"first-page": "429",
"journal-title": "Cell",
"key": "1780_CR25",
"unstructured": "Hou, Y. J. et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182, 429–446 (2020).",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1858177",
"author": "K Rosenke",
"doi-asserted-by": "publisher",
"first-page": "2673",
"journal-title": "Emerg. Microbes Infect.",
"key": "1780_CR26",
"unstructured": "Rosenke, K. et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 9, 2673–2684 (2020).",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2024671",
"author": "KS Corbett",
"doi-asserted-by": "publisher",
"first-page": "1544",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR27",
"unstructured": "Corbett, K. S. et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 383, 1544–1555 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2423-5",
"author": "BN Williamson",
"doi-asserted-by": "publisher",
"first-page": "273",
"journal-title": "Nature",
"key": "1780_CR28",
"unstructured": "Williamson, B. N. et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585, 273–276 (2020).",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2111477118",
"author": "R Ke",
"doi-asserted-by": "publisher",
"first-page": "e2111477118",
"journal-title": "Proc. Natl Acad. Sci. USA",
"key": "1780_CR29",
"unstructured": "Ke, R., Zitzmann, C., Ho, D. D., Ribeiro, R. M. & Perelson, A. S. In vivo kinetics of SARS-CoV-2 infection and its relationship with a person’s infectiousness. Proc. Natl Acad. Sci. USA 118, e2111477118 (2021).",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2027040",
"author": "MC Kim",
"doi-asserted-by": "publisher",
"first-page": "671",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR30",
"unstructured": "Kim, M. C. et al. Duration of culturable SARS-CoV-2 in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 671–673 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2001737",
"author": "L Zou",
"doi-asserted-by": "publisher",
"first-page": "1177",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR31",
"unstructured": "Zou, L. et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 382, 1177–1179 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa077",
"author": "P Yu",
"doi-asserted-by": "publisher",
"first-page": "1757",
"journal-title": "J. Infect. Dis.",
"key": "1780_CR32",
"unstructured": "Yu, P., Zhu, J., Zhang, Z. & Han, Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J. Infect. Dis. 221, 1757–1761 (2020).",
"volume": "221",
"year": "2020"
},
{
"DOI": "10.7326/M20-0504",
"author": "SA Lauer",
"doi-asserted-by": "publisher",
"first-page": "577",
"journal-title": "Ann. Intern. Med.",
"key": "1780_CR33",
"unstructured": "Lauer, S. A. et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 172, 577–582 (2020).",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1177/1073858420956905",
"author": "R Butowt",
"doi-asserted-by": "publisher",
"first-page": "582",
"journal-title": "Neuroscientist",
"key": "1780_CR34",
"unstructured": "Butowt, R. & von Bartheld, C. S. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist 27, 582–603 (2020).",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR35",
"unstructured": "Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383, 1813–1826 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.7326/M21-0653",
"author": "A Barratt-Due",
"doi-asserted-by": "publisher",
"first-page": "1261",
"journal-title": "Ann. Intern. Med.",
"key": "1780_CR36",
"unstructured": "Barratt-Due, A. et al. Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomized trial. Ann. Intern. Med. 174, 1261–1269 (2021).",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "1780_CR37",
"unstructured": "Gottlieb, R. L. et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 386, 305–315 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/v12091045",
"author": "KA Eberhardt",
"doi-asserted-by": "publisher",
"first-page": "1045",
"journal-title": "Viruses",
"key": "1780_CR38",
"unstructured": "Eberhardt, K. A. et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients. Viruses 12, 1045 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"author": "J Fajnzylber",
"doi-asserted-by": "publisher",
"journal-title": "Nat. Commun.",
"key": "1780_CR39",
"unstructured": "Fajnzylber, J. et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 11, 5493 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30985-3",
"author": "M Marks",
"doi-asserted-by": "publisher",
"first-page": "629",
"journal-title": "Lancet Infect. Dis.",
"key": "1780_CR40",
"unstructured": "Marks, M. et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect. Dis. 21, 629–636 (2021).",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1183/13993003.02724-2020",
"author": "D Munker",
"doi-asserted-by": "publisher",
"first-page": "2002724",
"journal-title": "Eur. Respir. J.",
"key": "1780_CR41",
"unstructured": "Munker, D. et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur. Respir. J. 58, 2002724 (2021).",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.06.067",
"author": "KA Walsh",
"doi-asserted-by": "publisher",
"first-page": "357",
"journal-title": "J. Infect.",
"key": "1780_CR42",
"unstructured": "Walsh, K. A. et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 81, 357–371 (2020).",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1157",
"author": "S Baggio",
"doi-asserted-by": "publisher",
"first-page": "148",
"journal-title": "Clin. Infect. Dis.",
"key": "1780_CR43",
"unstructured": "Baggio, S. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in the upper respiratory tract of children and adults with early acute coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 73, 148–150 (2021).",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"author": "R Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"journal-title": "Nature",
"key": "1780_CR44",
"unstructured": "Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020).",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01575-4",
"author": "M Levine-Tiefenbrun",
"doi-asserted-by": "publisher",
"first-page": "2108",
"journal-title": "Nat. Med.",
"key": "1780_CR45",
"unstructured": "Levine-Tiefenbrun, M. et al. Viral loads of delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 27, 2108–2110 (2021).",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/S2468-2667(20)30308-X",
"author": "BJ Quilty",
"doi-asserted-by": "publisher",
"first-page": "e175",
"journal-title": "Lancet Public Health",
"key": "1780_CR46",
"unstructured": "Quilty, B. J. et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. Lancet Public Health 6, e175–e183 (2021).",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.2147/CLEP.S311977",
"author": "I Petersen",
"doi-asserted-by": "publisher",
"first-page": "935",
"journal-title": "Clin. Epidemiol.",
"key": "1780_CR47",
"unstructured": "Petersen, I., Crozier, A., Buchan, I., Mina, M. J. & Bartlett, J. W. Recalibrating SARS-CoV-2 antigen rapid lateral flow test relative sensitivity from validation studies to absolute sensitivity for indicating individuals shedding transmissible virus. Clin. Epidemiol. 13, 935–940 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1186/s12916-020-01670-2",
"author": "D Gbesemete",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med.",
"key": "1780_CR48",
"unstructured": "Gbesemete, D. et al. Exploring the acceptability of controlled human infection with SARSCoV2—a public consultation. BMC Med. 18, 209 (2020).",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.12688/wellcomeopenres.17516.1",
"doi-asserted-by": "publisher",
"key": "1780_CR49",
"unstructured": "Barker, C. et al. Public attitudes to a human challenge study with SARS-CoV-2: a mixed-methods study. Preprint at https://doi.org/10.12688/wellcomeopenres.17516.1 (2022)."
},
{
"DOI": "10.1136/bmj.m3731",
"author": "AK Clift",
"doi-asserted-by": "publisher",
"first-page": "m3731",
"journal-title": "BMJ",
"key": "1780_CR50",
"unstructured": "Clift, A. K. et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ 371, m3731 (2020).",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.3758/BF03210709",
"author": "RL Doty",
"doi-asserted-by": "publisher",
"first-page": "381",
"journal-title": "Percept. Psychophys.",
"key": "1780_CR51",
"unstructured": "Doty, R. L., Frye, R. E. & Agrawal, U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept. Psychophys. 45, 381–384 (1989).",
"volume": "45",
"year": "1989"
},
{
"key": "1780_CR52",
"unstructured": "Coronavirus Clinical Characterisation Consortium. Site set-up. ISARIC 4C https://isaric4c.net/protocols"
},
{
"key": "1780_CR53",
"unstructured": "Center for Disease Control and Prevention. CDC’s Diagnostic Test for COVID-19 Only and Supplies. COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html (2021)."
},
{
"key": "1780_CR54",
"unstructured": "Zar, J. H. Biostatistical Analysis 5th edn (Pearson, 2010)."
},
{
"DOI": "10.1093/biomet/73.1.13",
"author": "K-Y Liang",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Biometrika",
"key": "1780_CR55",
"unstructured": "Liang, K.-Y. & Zeger, S. L. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22 (1986).",
"volume": "73",
"year": "1986"
}
],
"reference-count": 55,
"references-count": 55,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-1121993/v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41591-022-01780-9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "28"
}