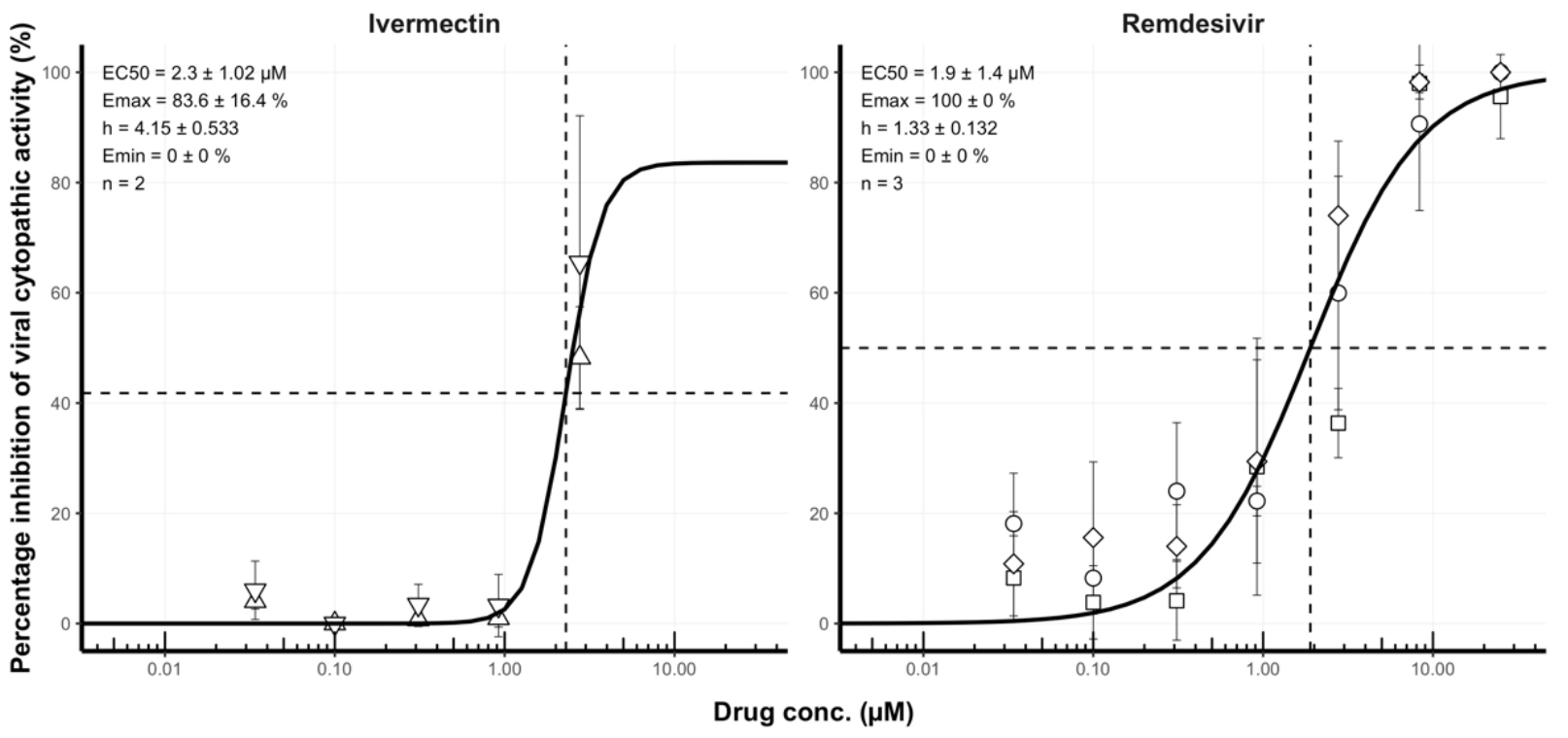
Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542, Dec 2020 (preprint)
In vitro study showing enhanced antiviral activity of ivermectin and remdesivir in combination.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Study covers ivermectin and remdesivir.
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Jeffreys et al., 24 Dec 2020, peer-reviewed, 17 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Remdesivir–ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2
International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2022.106542
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS
References
Agostini, Andres, Sims, Graham, Sheahan et al., Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease, mBio
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin Pharmacol Ther
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Berenbaum, A method for testing for synergy with any number of agents, J Infect Dis
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Consortium, Whost, Pan, Peto, Henao-Restrepo et al., Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Crump, Omura, Ivermectin, 'wonder drug' from Japan: the human use perspective, Proc Jpn Acad Ser B Phys Biol Sci
De Melo, Lazarini, Larrous, Feige, Kergoat et al., Anti-COVID-19 efficacy of ivermectin in the golden hamster, bioRxiv, doi:10.1101/2020.11.21.392639:2020.11.21.392639
Gonzalez-Paz, Hurtado-Leon, Lossada, Fernandez-Materan, Vera-Villalobos et al., Comparative study of the interaction of ivermectin with proteins of interest associated with SARS-CoV-2: A computational and biophysical approach, Biophys Chem
Greco, Bravo, Parsons, The search for synergy: a critical review from a response surface perspective, Pharmacol Rev
Heidary, Gharebaghi, Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen, J Antibiot
Humeniuk, Mathias, Cao, Osinusi, Shen et al., Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects, Clin Transl Sci
Humeniuk, Mathias, Kirby, Lutz, Cao et al., Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of Remdesivir, a SARS-CoV-2 Replication Inhibitor, Clin Pharmacokinet
Ianevski, Giri, Aittokallio, SynergyFinder 2.0: visual analytics of multi-drug combination synergies, Nucleic Acids Res
Ianevski, Yao, Biza, Zusinaite, Mannik et al., Identification and Tracking of Antiviral Drug Combinations, Viruses
Jermain, Hanafin, Cao, Lifschitz, Lanusse et al., Development of a Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung Exposure in Humans Following Oral Administration of Ivermectin for COVID-19
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, N Engl J Med
Kaptein, Jacobs, Langendries, Seldeslachts, Horst et al., Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci U S A
Klotz, Ogbuokiri, Okonkwo, Ivermectin binds avidly to plasma proteins, Eur J Clin Pharmacol
Mckee, Sternberg, Stange, Laufer, Naujokat, Candidate drugs against SARS-CoV-2 and COVID-19, Pharmacol Res
Odds, Synergy, antagonism, and what the chequerboard puts between them, J Antimicrob Chemother
Pizzorno, Padey, Dubois, Julien, Traversier et al., In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2, Antiviral Res
Riva, Yuan, Yin, Martin-Sancho, Matsunaga et al., Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing, Nature
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review, JAMA
Smit, Ochomo, Waterhouse, Kwambai, Abong et al., Pharmacokinetics-Pharmacodynamics of High-Dose Ivermectin with Dihydroartemisinin-Piperaquine on Mosquitocidal Activity and QT-Prolongation (IVERMAL), Clin Pharmacol Ther
Steinhoff, Vocanson, Voegel, Hacini-Rachinel, Schafer, Topical Ivermectin 10 mg/g and Oral Doxycycline 40 mg Modified-Release: Current Evidence on the Complementary Use of Anti-Inflammatory Rosacea Treatments, Adv Ther
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J
Who, WHO Coronovirus Disease (COVID-19) Dashboard
DOI record:
{
"DOI": "10.1016/j.ijantimicag.2022.106542",
"ISSN": [
"0924-8579"
],
"URL": "http://dx.doi.org/10.1016/j.ijantimicag.2022.106542",
"alternative-id": [
"S0924857922000309"
],
"article-number": "106542",
"author": [
{
"affiliation": [],
"family": "Jeffreys",
"given": "Laura N",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7160-6275",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pennington",
"given": "Shaun H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duggan",
"given": "Jack",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caygill",
"given": "Claire H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopeman",
"given": "Rose C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breen",
"given": "Alastair F",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8711-0290",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jinks",
"given": "Jessica B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ardrey",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donnellan",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patterson",
"given": "Edward I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hughes",
"given": "Grant L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "David W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O'Neill",
"given": "Paul M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aljayyoussi",
"given": "Ghaith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2331-3192",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ward",
"given": "Stephen A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biagini",
"given": "Giancarlo A",
"sequence": "additional"
}
],
"container-title": [
"International Journal of Antimicrobial Agents"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
31
]
],
"date-time": "2022-01-31T09:01:49Z",
"timestamp": 1643619709000
},
"deposited": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T04:40:46Z",
"timestamp": 1643690446000
},
"indexed": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T13:40:41Z",
"timestamp": 1643809241047
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0924-8579"
}
],
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 25,
"start": {
"date-parts": [
[
2022,
1,
26
]
],
"date-time": "2022-01-26T00:00:00Z",
"timestamp": 1643155200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857922000309?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857922000309?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "106542",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"International Journal of Antimicrobial Agents"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": [
"Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2"
],
"type": "journal-article"
}
jeffreys