Longitudinal patient-reported outcome trajectories in Long COVID: Findings from the STOP-PASC Clinical Trial
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf634, STOP-PASC, NCT05576662, Oct 2025
Secondary analysis of NCT0557666 (history). RCT 155 adults with long COVID showing no benefit from a 15-day course of paxlovid. Patients with worsening symptoms were more likely to be treated with paxlovid, without statistical significance.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of progression, 127.2% higher, RR 2.27, p = 0.14, treatment 18 of 101 (17.8%), control 4 of 51 (7.8%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jagannathan et al., 8 Oct 2025, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 10 authors, study period 8 November, 2022 - 12 September, 2023, trial NCT05576662 (history) (STOP-PASC).
Contact: hbonilla@stanford.edu, prasj@stanford.edu.
Longitudinal patient-reported outcome trajectories in Long COVID: Findings from the STOP-PASC Clinical Trial
Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf634
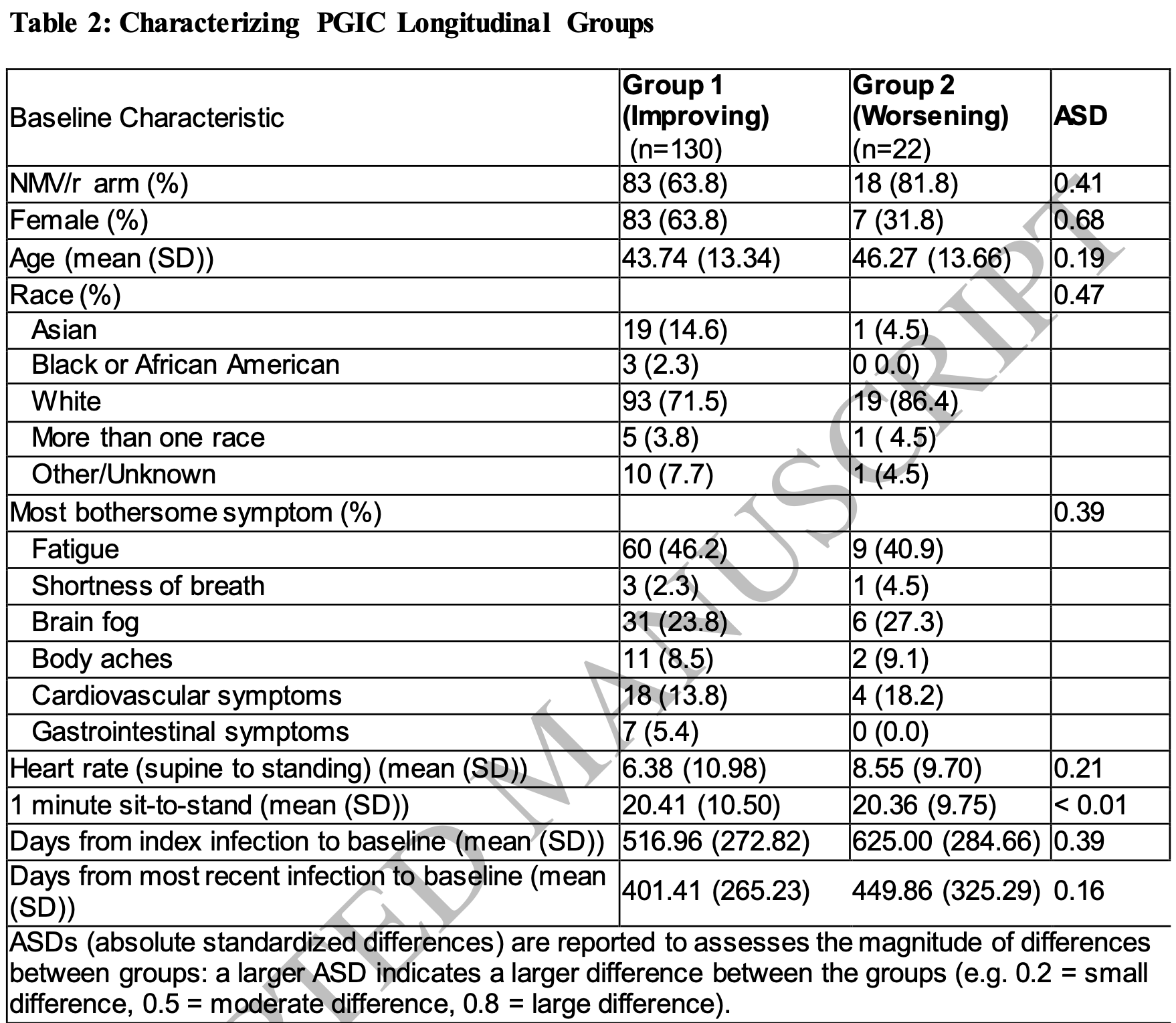
Background: Long COVID is a heterogeneous post-infectious condition. Although patientreported outcome (PRO) measures for diagnosis or therapeutic monitoring have been adapted from related complex chronic illnesses, no PRO has been validated specifically in Long COVID. The STOP-PASC randomized, placebo-controlled trial of nirmatrelvir/ritonavir (NMV/r) in adults with Long COVID showed no overall treatment effect. This exploratory analysis aimed to identify distinct symptom trajectories and clinical characteristics associated with improvement or worsening over time.
Methods: We performed latent class trajectory modeling (LCTM) on PRO measuresincluding the Patient Global Impression of Severity (PGIS), Patient Global Impression of Change (PGIC), PROMIS domains, and core symptoms-among 155 randomized participants. Participants were followed for 15 weeks with serial symptom assessments. Trajectory groups were identified using Bayesian Information Criteria and characterized using descriptive statistics and absolute standardized differences.
References
Barilaite, Watson, Hocaoglu, Understanding Patient-Reported Outcome Measures Used in Adult Survivors Experiencing Long-Term Effects After COVID-19 Infection: A Rapid Review, J Patient Cent Res Rev. Spring
Cella, Yount, Rothrock, The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years, Med Care
Cohen, Jaudon, Schurman, Impact of extended-course oral nirmatrelvir/ritonavir in established Long COVID: a case series, Commun Med (Lond)
Ejalonibu, Amah, Aburub, Kumar, Frederick et al., A review of Patient Reported Outcome Measures (PROMs) for characterizing Long COVID (LC)-merits, gaps, and recommendations, J Patient Rep Outcomes
Ely, Brown, Fineberg, National Academies of Sciences E, Medicine Committee on Examining the Working Definition for Long C. Long Covid Defined, N Engl J Med
Fung, Baye, Baik, Mcdonald, Nirmatrelvir and Molnupiravir and Post-COVID-19 Condition in Older Patients, JAMA Intern Med
Geng, Bonilla, Hedlin, Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial, JAMA Intern Med
Hartung, Bahmer, Chaplinskaya-Sobol, Predictors of non-recovery from fatigue and cognitive deficits after COVID-19: a prospective, longitudinal, population-based study, EClinicalMedicine
Khullar, Zhang, Zang, Racial/Ethnic Disparities in Post-acute Sequelae of SARS-CoV-2 Infection in New York: an EHR-Based Cohort Study from the RECOVER Program, J. Gen. Intern. Med
Lennon, Kelly, Sperrin, Framework to construct and interpret latent class trajectory modelling, BMJ Open
Lennon, Watson, LCTMtools}: Latent Class Trajectory Models tools R Functions
Monti, Tamayo, Mesirov, Golub, Consensus Clustering: A Resampling-Based Method for Class Discovery and Visualization of Gene Expression Microarray Data, Machine Learning
Proust-Lima, Philipps, Liquet, Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm, Journal of Statistical Software
Proust-Lima, Philipps, Liquet, lcmm: Extended Mixed Models Using Latent Classes and Latent Processes
Sawano, Bhattacharjee, Caraballo, Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebocontrolled, phase 2, decentralised trial, Lancet Infect Dis
Vahratian, Saydah, Bertolli, Unger, Co, Prevalence of Post-COVID-19 Condition and Activity-Limiting Post-COVID-19 Condition Among Adults, JAMA Netw Open
Wang, Zang, Li, Real-World Effectiveness of Nirmatrelvir in Protecting Long COVID for Outpatient Adult Patients -A Large-Scale Observational Cohort Study from the RECOVER Initiative, Res Sq
Who, Global COVID-19 Clinical Platform Case Report Form (CRF) for Post COVID condition (Post COVID-19 CRF)
Xie, Choi, Al-Aly, Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition, JAMA Intern Med
DOI record:
{
"DOI": "10.1093/ofid/ofaf634",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf634",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Long COVID is a heterogeneous post-infectious condition. Although patient-reported outcome (PRO) measures for diagnosis or therapeutic monitoring have been adapted from related complex chronic illnesses, no PRO has been validated specifically in Long COVID. The STOP-PASC randomized, placebo-controlled trial of nirmatrelvir/ritonavir (NMV/r) in adults with Long COVID showed no overall treatment effect. This exploratory analysis aimed to identify distinct symptom trajectories and clinical characteristics associated with improvement or worsening over time.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed latent class trajectory modeling (LCTM) on PRO measures—including the Patient Global Impression of Severity (PGIS), Patient Global Impression of Change (PGIC), PROMIS domains, and core symptoms—among 155 randomized participants. Participants were followed for 15 weeks with serial symptom assessments. Trajectory groups were identified using Bayesian Information Criteria and characterized using descriptive statistics and absolute standardized differences.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>LCTM revealed heterogeneity in symptom trajectories. Two groups emerged for PGIS (improving n=17, persistent/severe n=136) and PGIC (improving n=130; worsening n=22). PROMIS-Physical Function modeling identified four groups (improving, normal/mild, moderate, and severe), fatigue core symptom modeling identified three (improving; moderate; severe). Worsening groups had higher proportions of NMV/r-treated participants and greater prevalence of cardiovascular symptoms and low-dose naltrexone use. Improving groups had shorter time since infection and higher baseline physical function. No subgroup showed a clear benefit from NMV/r.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Distinct PRO trajectories reflect the clinical heterogeneity of Long COVID. NMV/r showed no clear benefit across subgroups. These findings emphasize the need for validated, Long COVID-specific PRO instruments and targeted therapeutic trials tailored to Long COVID subtypes.</jats:p>\n </jats:sec>",
"article-number": "ofaf634",
"author": [
{
"ORCID": "https://orcid.org/0000-0001-6305-758X",
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine , Stanford, California"
}
],
"authenticated-orcid": false,
"family": "Jagannathan",
"given": "Prasanna",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-0757-7959",
"affiliation": [
{
"name": "Stanford Quantitative Sciences Unit , Stanford, California"
}
],
"authenticated-orcid": false,
"family": "Hedlin",
"given": "Haley",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2302-3809",
"affiliation": [
{
"name": "Stanford Quantitative Sciences Unit , Stanford, California"
},
{
"name": "Division of Research, Kaiser Permanente Northern California, Pleasanton , California"
}
],
"authenticated-orcid": false,
"family": "Liang",
"given": "Jane W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Quantitative Sciences Unit , Stanford, California"
}
],
"family": "Shaw",
"given": "Blake",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine , Stanford, California"
}
],
"family": "Maestri",
"given": "Evan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2824-0893",
"affiliation": [
{
"name": "Stanford Quantitative Sciences Unit , Stanford, California"
}
],
"authenticated-orcid": false,
"family": "Lin",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine , Stanford, California"
}
],
"family": "Utz",
"given": "P J",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0630-0306",
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine , Stanford, California"
},
{
"name": "Department of Internal Medicine, University of Iowa Carver College of Medicine , Iowa City, Iowa"
}
],
"authenticated-orcid": false,
"family": "Singh",
"given": "Upinder",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9891-5705",
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine , Stanford, California"
}
],
"authenticated-orcid": false,
"family": "Geng",
"given": "Linda N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine , Stanford, California"
}
],
"family": "Bonilla",
"given": "Hector",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
10,
8
]
],
"date-time": "2025-10-08T04:58:53Z",
"timestamp": 1759899533000
},
"deposited": {
"date-parts": [
[
2025,
10,
8
]
],
"date-time": "2025-10-08T04:58:53Z",
"timestamp": 1759899533000
},
"indexed": {
"date-parts": [
[
2025,
10,
8
]
],
"date-time": "2025-10-08T05:40:40Z",
"timestamp": 1759902040272,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
10,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
8
]
],
"date-time": "2025-10-08T00:00:00Z",
"timestamp": 1759881600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaf634/64540869/ofaf634.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaf634/64540869/ofaf634.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
10,
8
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
8
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofaf634/8277127"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Longitudinal patient-reported outcome trajectories in Long COVID: Findings from the STOP-PASC Clinical Trial",
"type": "journal-article"
}