Tocilizumab in patients with severe COVID-19: a retrospective cohort study
et al., The Lancet Rheumatology, doi:10.1016/S2665-9913(20)30173-9, Aug 2020
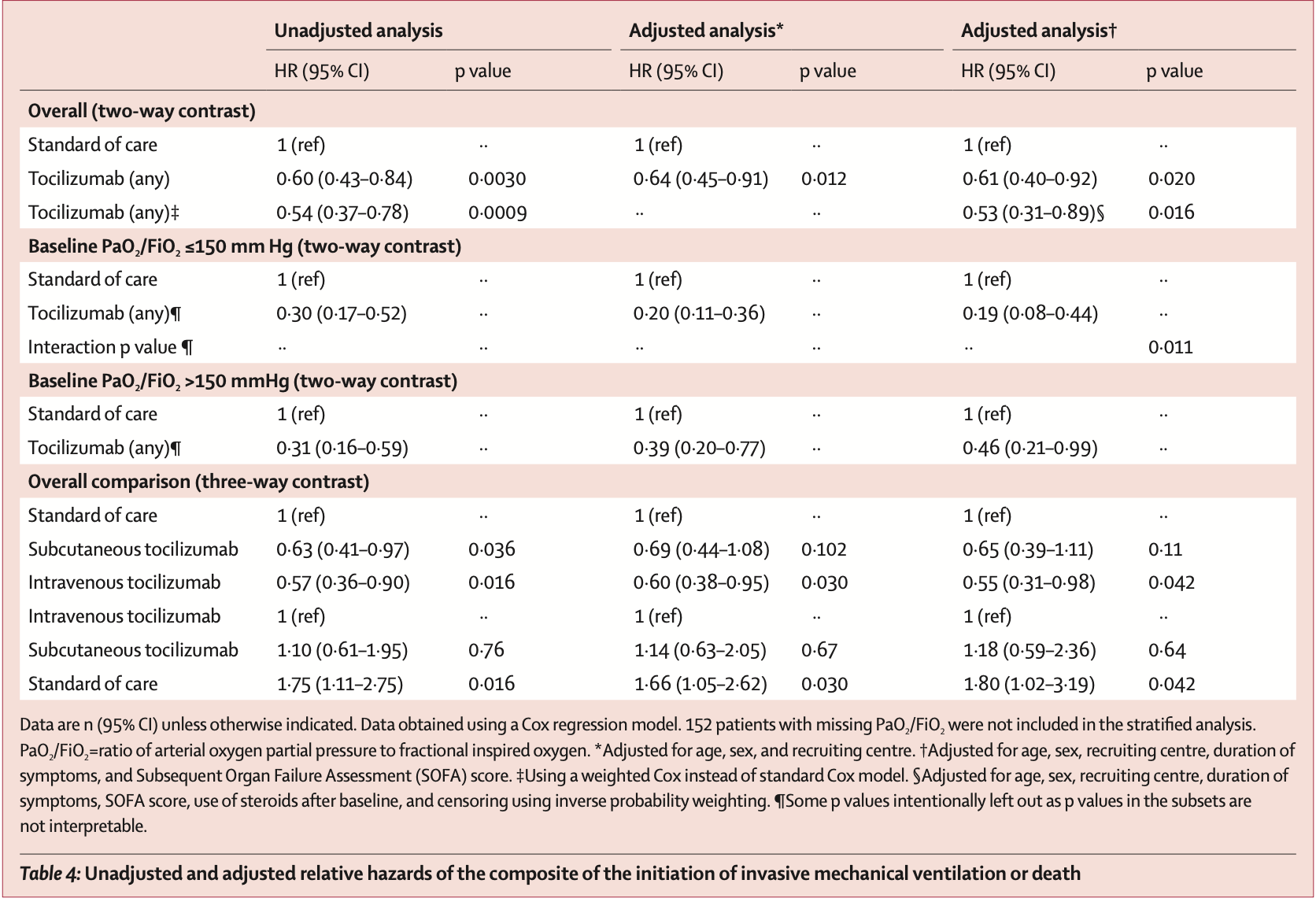
Retrospective 544 patients with severe COVID-19 pneumonia showing lower mechanical ventilation or death with tocilizumab.
|
risk of death, 62.0% lower, HR 0.38, p = 0.01, treatment 13 of 125 (10.4%), control 73 of 179 (40.8%), NNT 3.3, adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of death/intubation, 39.0% lower, HR 0.61, p = 0.02, treatment 125, control 179, adjusted per study, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Guaraldi et al., 31 Aug 2020, retrospective, Italy, peer-reviewed, median age 67.0, 34 authors, study period 21 February, 2020 - 24 March, 2020.
Contact: cristina.mussini@unimore.it.
Tocilizumab in patients with severe COVID-19: a retrospective cohort study
The Lancet Rheumatology, doi:10.1016/s2665-9913(20)30173-9
Background No therapy is approved for COVID-19 pneumonia. The aim of this study was to assess the role of tocilizumab in reducing the risk of invasive mechanical ventilation and death in patients with severe COVID-19 pneumonia who received standard of care treatment. Methods This retrospective, observational cohort study included adults (≥18 years) with severe COVID-19 pneumonia who were admitted to tertiary care centres in Bologna and Reggio Emilia, Italy, between Feb 21 and March 24, 2020, and a tertiary care centre in Modena, Italy, between Feb 21 and April 30, 2020. All patients were treated with the standard of care (ie, supplemental oxygen, hydroxychloroquine, azithromycin, antiretrovirals, and low molecular weight heparin), and a non-randomly selected subset of patients also received tocilizumab. Tocilizumab was given either intravenously at 8 mg/kg bodyweight (up to a maximum of 800 mg) in two infusions, 12 h apart, or subcutaneously at 162 mg administered in two simultaneous doses, one in each thigh (ie, 324 mg in total), when the intravenous formulation was unavailable. The primary endpoint was a composite of invasive mechanical ventilation or death. Treatment groups were compared using Kaplan-Meier curves and Cox regression analysis after adjusting for sex, age, recruiting centre, duration of symptoms, and baseline Sequential Organ Failure Assessment (SOFA) score. Findings Of 1351 patients admitted, 544 (40%) had severe COVID-19 pneumonia and were included in the study. 57 (16%) of 365 patients in the standard care group needed mechanical ventilation, compared with 33 (18%) of 179 patients treated with tocilizumab (p=0•41; 16 [18%] of 88 patients treated intravenously and 17 [19%] of 91 patients treated subcutaneously). 73 (20%) patients in the standard care group died, compared with 13 (7%; p<0•0001) patients treated with tocilizumab (six [7%] treated intravenously and seven [8%] treated subcutaneously). After adjustment for sex, age, recruiting centre, duration of symptoms, and SOFA score, tocilizumab treatment was associated with a reduced risk of invasive mechanical ventilation or death (adjusted hazard ratio 0•61, 95% CI 0•40-0•92; p=0•020). 24 (13%) of 179 patients treated with tocilizumab were diagnosed with new infections, versus 14 (4%) of 365 patients treated with standard of care alone (p<0•0001). Interpretation Treatment with tocilizumab, whether administered intravenously or subcutaneously, might reduce the risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia.
References
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Cavalli, Luca, De, Campochiaro, Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheumatol
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis
Davidson, Banham, Elliott, BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults, Thorax
Dostalek, Gardner, Gurbaxani, Rose, Chetty, Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies, Clin Pharmacokinet
Gill, Machavaram, Rose, Chetty, Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics, Clin Pharmacokinet
Grasselli, Zangrillo, Zanella, Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy, JAMA
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Hennigan, Kavanaugh, Interleukin-6 inhibitors in the treatment of rheumatoid arthritis, Ther Clin Risk Manag
Higgs, Mcgrath, Goddard, Guidelines for the management of tracheal intubation in critically ill adults, Br J Anaesth
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jain, Palta, Saroa, Palta, Sama et al., Sequential organ failure assessment scoring and prediction of patient's outcome in Intensive Care Unit of a tertiary care hospital, J Anaesthesiol Clin Pharmacol
Klopfenstein, Zayet, Lohse, Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients, Med Mal Infect, doi:10.1016/j.medmal.2020.05.001
Kotch, Barrett, Teachey, Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome, Expert Rev Clin Immunol
Le, Li, Yuan, FDA approval summary: tocilizumab for treatment of chimeric antigen receptor t cell-induced severe or life-threatening cytokine release syndrome, Oncologist
Lodi, Phillips, Lundgren, Effect estimates in randomized trials and observational studies: comparing apples with apples, Am J Epidemiol
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: a single center experience, J Med Virol, doi:10.1002/jmv.25801
Meng, Wu, Lu, Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients, PLoS Pathog
Nicastri, 'abramo, Faggioni, Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020, Euro Surveill
Norelli, Camisa, Barbiera, Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells, Nat Med
Onder, Rezza, Brusaferro, Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy, JAMA, doi:10.1001/jama.2020.4683
Pedersen, Ho, SARS-CoV-2: a storm is raging, J Clin Invest
Radbel, Narayanan, Bhatt, Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: a cautionary case report, Chest, doi:10.1016/j.chest.2020.04.024
Review, Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID-19 pneumonia
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet
Sciascia, Aprà, Baffa, Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19, Clin Exp Rheumatol
Scott, Tocilizumab: a review in rheumatoid arthritis, Drugs
Shang, Zhao, Hu, Du, Cao, On the use of corticosteroids for 2019-nCoV pneumonia, Lancet
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant
Stebbing, Phelan, Griffin, COVID-19: combining antiviral and anti-inflammatory treatments, Lancet Infect Dis
Stone, Tuckwell, Dimonaco, Trial of tocilizumab in giant-cell arteritis, N Engl J Med
Swerdlow, Holmes, Kuchenbaecker, The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis, Lancet
Toniati, Piva, Cattalini, Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy, Autoimmun Rev, doi:10.1016/j.autrev.2020.102568
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
Zhang, Rowell, Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects, Int J Clin Pharmacol Ther
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1016/s2665-9913(20)30173-9",
"ISSN": [
"2665-9913"
],
"URL": "http://dx.doi.org/10.1016/S2665-9913(20)30173-9",
"alternative-id": [
"S2665991320301739"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Tocilizumab in patients with severe COVID-19: a retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Rheumatology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2665-9913(20)30173-9"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2665-9913(20)30210-1"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Guaraldi",
"given": "Giovanni",
"sequence": "first"
},
{
"affiliation": [],
"family": "Meschiari",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cozzi-Lepri",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milic",
"given": "Jovana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tonelli",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Menozzi",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Franceschini",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cuomo",
"given": "Gianluca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orlando",
"given": "Gabriella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borghi",
"given": "Vanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santoro",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Di Gaetano",
"given": "Margherita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puzzolante",
"given": "Cinzia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carli",
"given": "Federica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bedini",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Corradi",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fantini",
"given": "Riccardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castaniere",
"given": "Ivana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tabbì",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Girardis",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tedeschi",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giannella",
"given": "Maddalena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bartoletti",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pascale",
"given": "Renato",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dolci",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brugioni",
"given": "Lucio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pietrangelo",
"given": "Antonello",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cossarizza",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pea",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clini",
"given": "Enrico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvarani",
"given": "Carlo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Massari",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viale",
"given": "Pier Luigi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mussini",
"given": "Cristina",
"sequence": "additional"
}
],
"container-title": "The Lancet Rheumatology",
"container-title-short": "The Lancet Rheumatology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
6,
24
]
],
"date-time": "2020-06-24T22:31:48Z",
"timestamp": 1593037908000
},
"deposited": {
"date-parts": [
[
2020,
9,
3
]
],
"date-time": "2020-09-03T20:40:23Z",
"timestamp": 1599165623000
},
"indexed": {
"date-parts": [
[
2025,
6,
7
]
],
"date-time": "2025-06-07T18:24:56Z",
"timestamp": 1749320696674
},
"is-referenced-by-count": 728,
"issue": "8",
"issued": {
"date-parts": [
[
2020,
8
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2020,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
8,
1
]
],
"date-time": "2020-08-01T00:00:00Z",
"timestamp": 1596240000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2665991320301739?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2665991320301739?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e474-e484",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
8
]
]
},
"published-print": {
"date-parts": [
[
2020,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/S2665-9913(20)30173-9_bib1",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4683",
"article-title": "Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy",
"author": "Onder",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/S2665-9913(20)30173-9_bib2",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/S2665-9913(20)30173-9_bib3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/S2665-9913(20)30173-9_bib4",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.11.2000230",
"article-title": "Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020",
"author": "Nicastri",
"doi-asserted-by": "crossref",
"journal-title": "Euro Surveill",
"key": "10.1016/S2665-9913(20)30173-9_bib5",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"article-title": "COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal",
"author": "Siddiqi",
"doi-asserted-by": "crossref",
"first-page": "405",
"journal-title": "J Heart Lung Transplant",
"key": "10.1016/S2665-9913(20)30173-9_bib6",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/S2665-9913(20)30173-9_bib7",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1172/JCI137647",
"article-title": "SARS-CoV-2: a storm is raging",
"author": "Pedersen",
"doi-asserted-by": "crossref",
"first-page": "2202",
"journal-title": "J Clin Invest",
"key": "10.1016/S2665-9913(20)30173-9_bib8",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "10.1016/S2665-9913(20)30173-9_bib9",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1007/s40265-017-0829-7",
"article-title": "Tocilizumab: a review in rheumatoid arthritis",
"author": "Scott",
"doi-asserted-by": "crossref",
"first-page": "1865",
"journal-title": "Drugs",
"key": "10.1016/S2665-9913(20)30173-9_bib10",
"volume": "77",
"year": "2017"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"article-title": "COVID-19: combining antiviral and anti-inflammatory treatments",
"author": "Stebbing",
"doi-asserted-by": "crossref",
"first-page": "400",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S2665-9913(20)30173-9_bib11",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.2147/TCRM.S3470",
"article-title": "Interleukin-6 inhibitors in the treatment of rheumatoid arthritis",
"author": "Hennigan",
"doi-asserted-by": "crossref",
"first-page": "767",
"journal-title": "Ther Clin Risk Manag",
"key": "10.1016/S2665-9913(20)30173-9_bib12",
"volume": "4",
"year": "2008"
},
{
"DOI": "10.1016/S0140-6736(12)60110-X",
"article-title": "The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis",
"author": "Swerdlow",
"doi-asserted-by": "crossref",
"first-page": "1214",
"journal-title": "Lancet",
"key": "10.1016/S2665-9913(20)30173-9_bib13",
"volume": "379",
"year": "2012"
},
{
"DOI": "10.1038/s41591-018-0036-4",
"article-title": "Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells",
"author": "Norelli",
"doi-asserted-by": "crossref",
"first-page": "739",
"journal-title": "Nat Med",
"key": "10.1016/S2665-9913(20)30173-9_bib14",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1080/1744666X.2019.1629904",
"article-title": "Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome",
"author": "Kotch",
"doi-asserted-by": "crossref",
"first-page": "813",
"journal-title": "Expert Rev Clin Immunol",
"key": "10.1016/S2665-9913(20)30173-9_bib15",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa1613849",
"article-title": "Trial of tocilizumab in giant-cell arteritis",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "N Engl J Med",
"key": "10.1016/S2665-9913(20)30173-9_bib16",
"volume": "377",
"year": "2017"
},
{
"DOI": "10.1002/jmv.25801",
"article-title": "Tocilizumab treatment in COVID-19: a single center experience",
"author": "Luo",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/S2665-9913(20)30173-9_bib17",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.04.024",
"article-title": "Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: a cautionary case report",
"author": "Radbel",
"doi-asserted-by": "crossref",
"journal-title": "Chest",
"key": "10.1016/S2665-9913(20)30173-9_bib18",
"year": "2020"
},
{
"article-title": "Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19",
"author": "Sciascia",
"first-page": "529",
"journal-title": "Clin Exp Rheumatol",
"key": "10.1016/S2665-9913(20)30173-9_bib19",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.medmal.2020.05.001",
"article-title": "Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients",
"author": "Klopfenstein",
"doi-asserted-by": "crossref",
"journal-title": "Med Mal Infect",
"key": "10.1016/S2665-9913(20)30173-9_bib20",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"article-title": "Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy",
"author": "Toniati",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun Rev",
"key": "10.1016/S2665-9913(20)30173-9_bib21",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2665-9913(20)30173-9_bib24",
"year": "2020"
},
{
"DOI": "10.1634/theoncologist.2018-0028",
"article-title": "FDA approval summary: tocilizumab for treatment of chimeric antigen receptor t cell-induced severe or life-threatening cytokine release syndrome",
"author": "Le",
"doi-asserted-by": "crossref",
"first-page": "943",
"journal-title": "Oncologist",
"key": "10.1016/S2665-9913(20)30173-9_bib25",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1007/s40262-015-0361-4",
"article-title": "Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics",
"author": "Gill",
"doi-asserted-by": "crossref",
"first-page": "789",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/S2665-9913(20)30173-9_bib26",
"volume": "55",
"year": "2016"
},
{
"DOI": "10.1007/s40262-012-0027-4",
"article-title": "Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies",
"author": "Dostalek",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/S2665-9913(20)30173-9_bib27",
"volume": "52",
"year": "2013"
},
{
"DOI": "10.5414/CP201819",
"article-title": "Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "443",
"journal-title": "Int J Clin Pharmacol Ther",
"key": "10.1016/S2665-9913(20)30173-9_bib28",
"volume": "51",
"year": "2013"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"article-title": "A new method of classifying prognostic comorbidity in longitudinal studies: development and validation",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "J Chronic Dis",
"key": "10.1016/S2665-9913(20)30173-9_bib29",
"volume": "40",
"year": "1987"
},
{
"DOI": "10.4103/0970-9185.168165",
"article-title": "Sequential organ failure assessment scoring and prediction of patient's outcome in Intensive Care Unit of a tertiary care hospital",
"author": "Jain",
"doi-asserted-by": "crossref",
"first-page": "364",
"journal-title": "J Anaesthesiol Clin Pharmacol",
"key": "10.1016/S2665-9913(20)30173-9_bib30",
"volume": "32",
"year": "2016"
},
{
"DOI": "10.1016/j.bja.2017.10.021",
"article-title": "Guidelines for the management of tracheal intubation in critically ill adults",
"author": "Higgs",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Br J Anaesth",
"key": "10.1016/S2665-9913(20)30173-9_bib31",
"volume": "120",
"year": "2018"
},
{
"DOI": "10.1136/thoraxjnl-2015-208209",
"article-title": "BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults",
"author": "Davidson",
"doi-asserted-by": "crossref",
"first-page": "ii1",
"issue": "suppl 2",
"journal-title": "Thorax",
"key": "10.1016/S2665-9913(20)30173-9_bib32",
"volume": "71",
"year": "2016"
},
{
"DOI": "10.1093/aje/kwz100",
"article-title": "Effect estimates in randomized trials and observational studies: comparing apples with apples",
"author": "Lodi",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Am J Epidemiol",
"key": "10.1016/S2665-9913(20)30173-9_bib33",
"volume": "188",
"year": "2019"
},
{
"DOI": "10.1016/S0140-6736(20)30317-2",
"article-title": "Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Lancet",
"key": "10.1016/S2665-9913(20)30173-9_bib35",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30361-5",
"article-title": "On the use of corticosteroids for 2019-nCoV pneumonia",
"author": "Shang",
"doi-asserted-by": "crossref",
"first-page": "683",
"journal-title": "Lancet",
"key": "10.1016/S2665-9913(20)30173-9_bib36",
"volume": "195",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"first-page": "e325",
"journal-title": "Lancet Rheumatol",
"key": "10.1016/S2665-9913(20)30173-9_bib37",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1574",
"journal-title": "JAMA",
"key": "10.1016/S2665-9913(20)30173-9_bib38",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1008520",
"article-title": "Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "PLoS Pathog",
"key": "10.1016/S2665-9913(20)30173-9_bib39",
"volume": "16",
"year": "2020"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2665991320301739"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tocilizumab in patients with severe COVID-19: a retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "2"
}