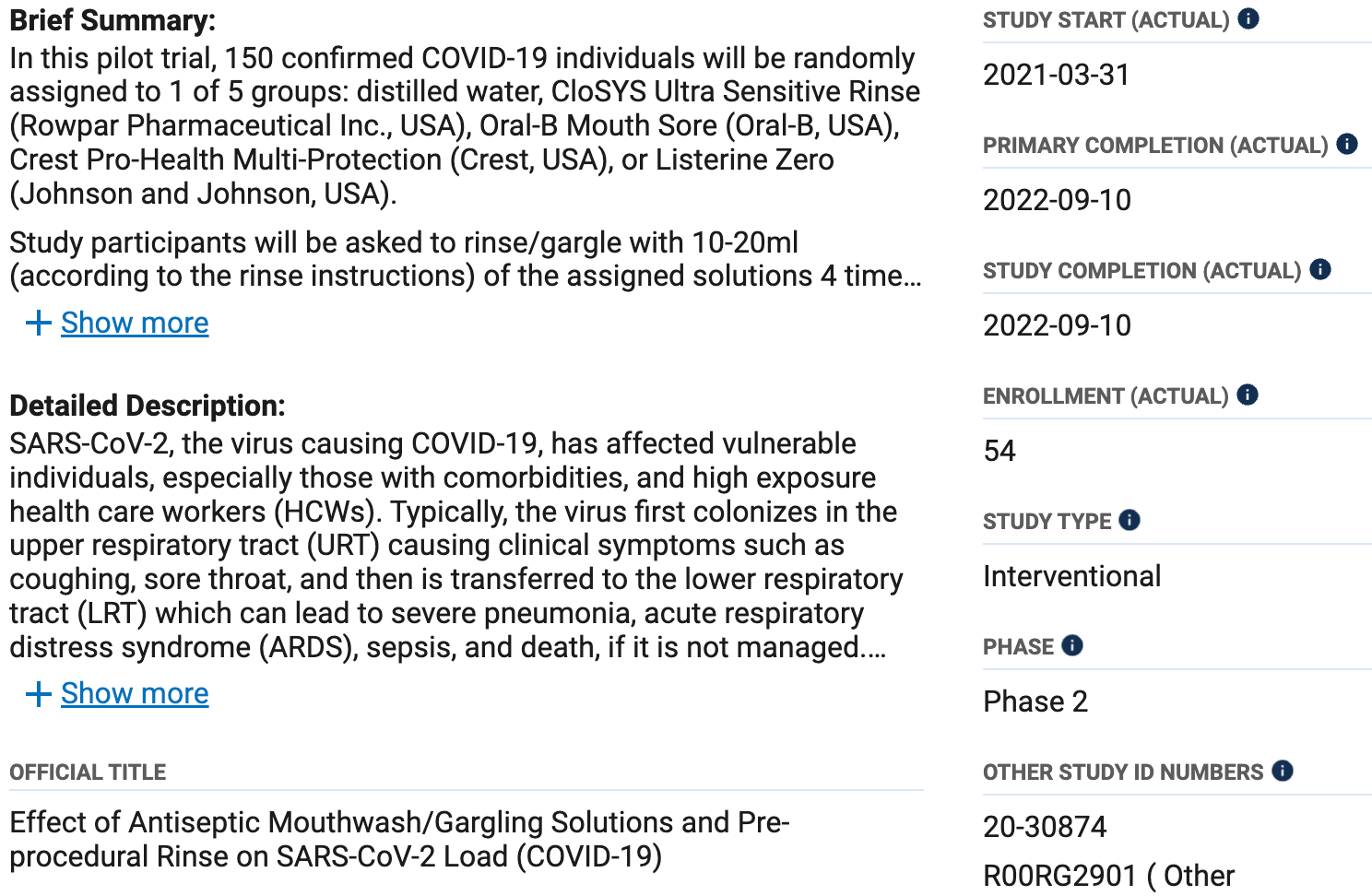
Antiseptic Mouthwash / Pre-Procedural Rinse on SARS-CoV-2 Load (COVID-19) (AMPoL)
et al., NCT04409873, AMPoL, NCT04409873, Nov 2023
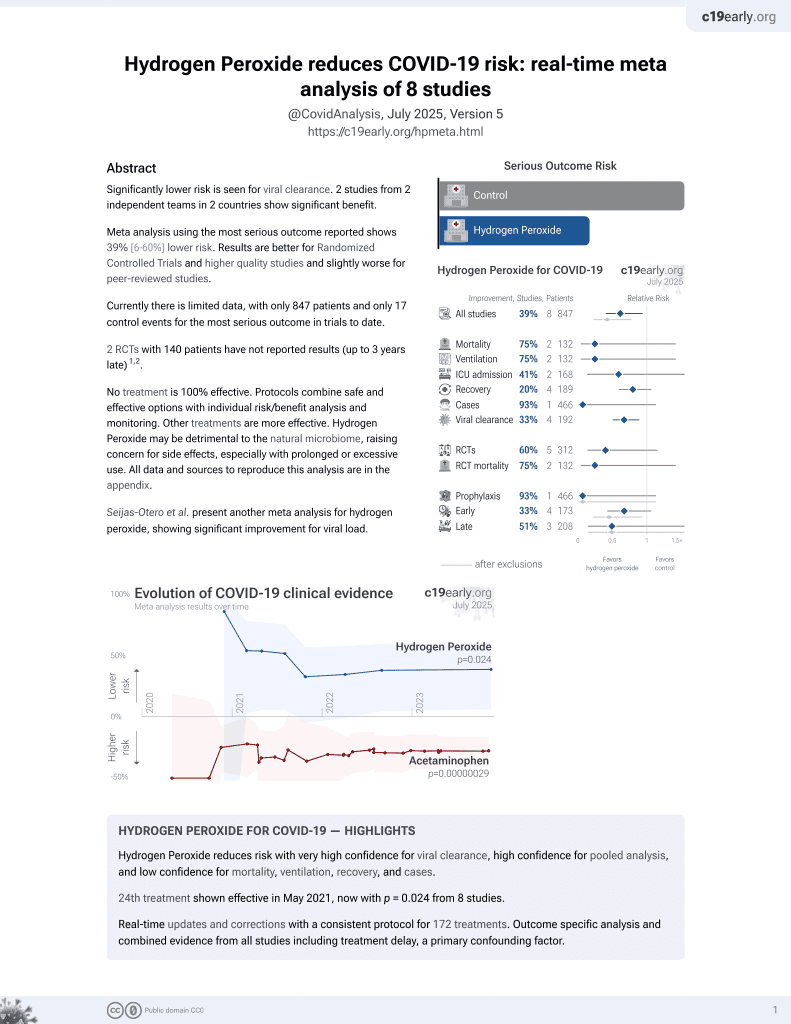
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Early terminated RCT with very limited information reported in the registry and only one patient showing symptoms. There is not enough information to assess the viral load results in the registry - the protocol indicates right-censoring for patients with undetectable viral load which may be the majority of patients at 4 weeks.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers hydrogen peroxide and cetylpyridinium chloride.
|
risk of no recovery, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 6 (0.0%), control 1 of 6 (16.7%), NNT 6.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gansky et al., 18 Nov 2023, Double Blind Randomized Controlled Trial, USA, preprint, 1 author, trial NCT04409873 (history) (AMPoL).
Contact: stuart.gansky@ucsf.edu, sepideh.banava@ucsf.edu.