A Double-blind, Randomized, Controlled Trial of ATI-450 in Patients With Moderate-severe COVID-19
et al., NCT04481685, NCT04481685, Jun 2025
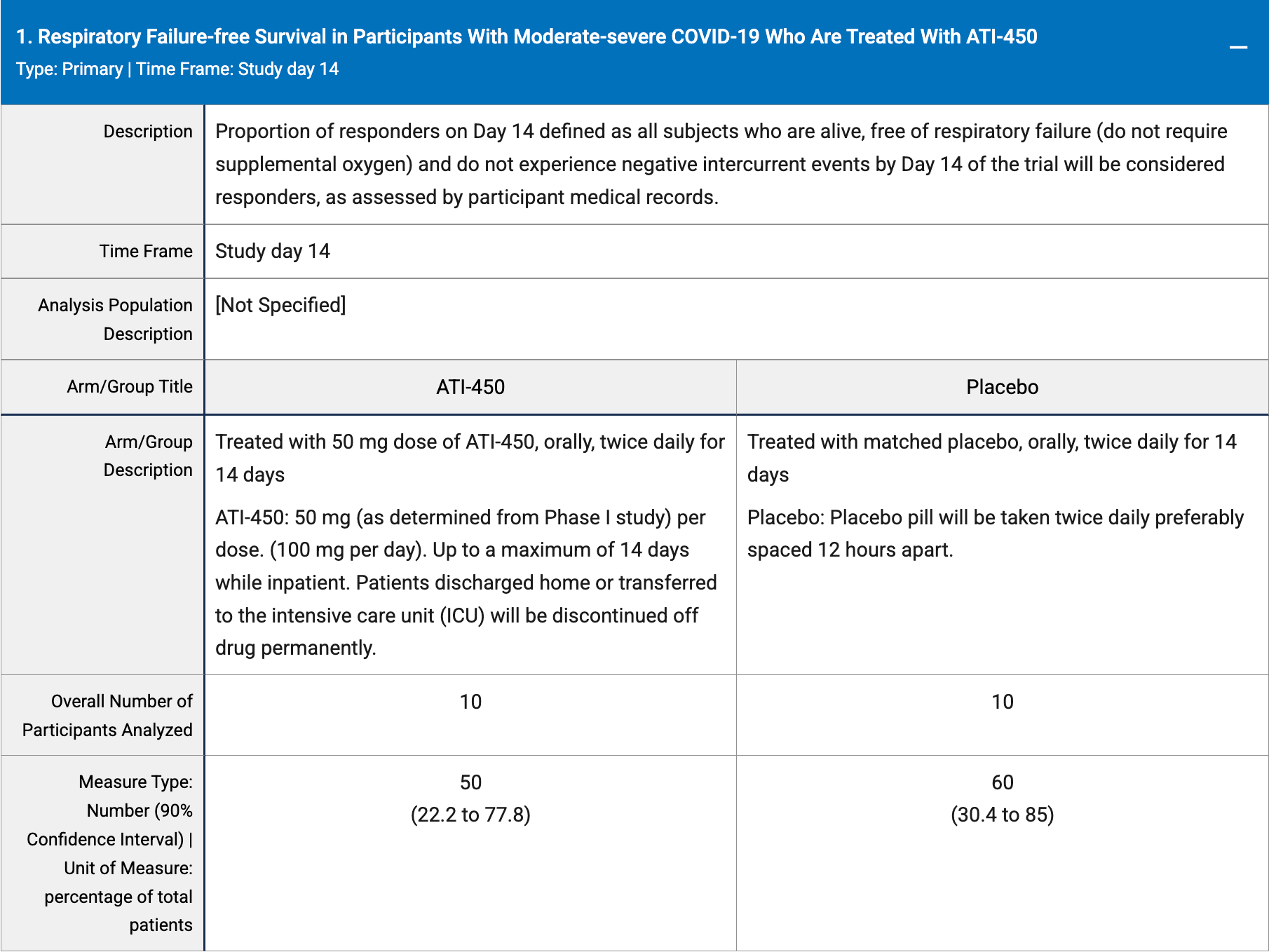
RCT 20 hospitalized patients showing no significant difference in outcomes with ATI-450 (zunsemetinib).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 1 of 10 (10.0%), control 1 of 10 (10.0%).
|
|
risk of progression, 25.0% higher, RR 1.25, p = 1.00, treatment 5 of 10 (50.0%), control 4 of 10 (40.0%), death or respiratory failure.
|
|
risk of progression, 100% higher, RR 2.00, p = 1.00, treatment 2 of 10 (20.0%), control 1 of 10 (10.0%), advanced respiratory care.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gan et al., 4 Jun 2025, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04481685 (history).
Contact: ggan@kumc.edu.