The Emergence of Escape Mutations in COVID-19 Following Anti-Spike Monoclonal Antibody Treatment: How Do We Tackle It?
et al., Infection and Drug Resistance, doi:10.2147/IDR.S540928, Oct 2025
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
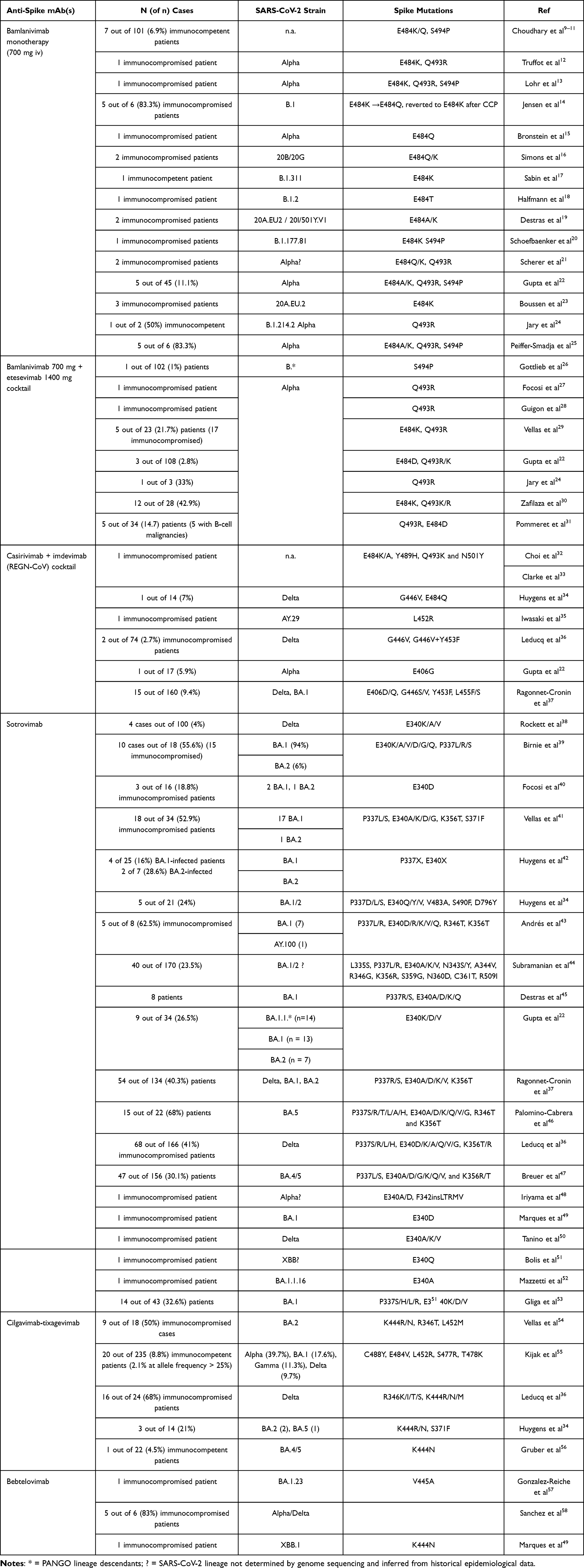
Review of treatment-emergent resistance to anti-spike monoclonal antibodies in COVID-19 patients. Monotherapies like bamlanivimab and sotrovimab showed very high resistance rates, while combination approaches had lower risk. The rapid evolution of the SARS-CoV-2 spike protein made it difficult for monoclonal antibody development to keep pace with natural selection, leading to baseline resistance against newer variants. Authors recommend against monoclonal antibody monotherapy in immunocompromised patients, and emphasize the importance of appropriate variant-sensitive monoclonal antibody selection and combining different antiviral mechanisms to prevent resistance.
Review covers tixagevimab/cilgavimab, casirivimab/imdevimab, bamlanivimab/etesevimab, sotrovimab, and bebtelovimab.
1.
Focosi et al., The Emergence of Escape Mutations in COVID-19 Following Anti-Spike Monoclonal Antibody Treatment: How Do We Tackle It?, Infection and Drug Resistance, doi:10.2147/IDR.S540928.
Focosi et al., 1 Oct 2025, Italy, peer-reviewed, 4 authors.
Contact: daniele.focosi@gmail.com.
The Emergence of Escape Mutations in COVID-19 Following Anti-Spike Monoclonal Antibody Treatment: How Do We Tackle It?
Infection and Drug Resistance, doi:10.2147/idr.s540928
Treatment-emergent resistance to anti-Spike monoclonal antibody (mAb) was a largely unexpected and dramatic finding along the COVID-19 pandemic. Emergence of resistant strains was particularly common in immunocompromised patients, who often harbored very high SARS-CoV-2 loads when treated with mAb monotherapies. Concerns were raised regarding the risk for some of those resistant variants to propagate in communities. In this review, we will summarize the experience thus far and suggest recommendations to prevent and manage mAb treatment-emergent resistance such as comboing and reliance over polyclonal immunoglobulins.
76 - 76 -
References
Ahiskali, Drekonja, Alpern, Conflicts of Interest Among Infectious Diseases Clinical Practice Guideline Authors and the Pharmaceutical Industry, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.8592
Andrés, González-Sánchez, Jiménez, Emergence of Delta and Omicron variants carrying resistance-associated mutations in immunocompromised patients undergoing sotrovimab treatment with long-term viral excretion, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.08.021
Bernasconi, Fanfoni, Alfonsi, Focosi, ConvMut: a Web tool to analyze viral convergent mutations along phylogenies, bioRxiv, doi:10.1101/2024.12.16.628620
Birnie, Biemond, Appelman, Development of Resistance-Associated Mutations After Sotrovimab Administration in High-risk Individuals Infected With the SARS-CoV-2 Omicron Variant, JAMA, doi:10.1001/jama.2022.13854
Bolis, Renteria, Alagna, SARS-CoV-2 genomic evolution during a severe and long-lasting omicron infection under antiviral therapy, BMC Infect Dis, doi:10.1186/s12879-025-10740-w
Boucau, Chew, Choudhary, Monoclonal antibody treatment drives rapid culture conversion in SARS-CoV-2 infection, Cell Rep Med, doi:10.1016/j.xcrm.2022.100678
Boussen, Salmona, De Fontbrune, Failure of bamlanivimab with selection of E484K mutation in an allogeneic stem cell transplant recipient with nosocomial SARS-CoV-2 infection, Antiviral Ther, doi:10.1177/13596535221097495
Bower, Karsany, Adam, Kankasha" in Kassala: a prospective observational cohort study of the clinical characteristics, epidemiology, genetic origin, and chronic impact of the 2018 epidemic of Chikungunya virus infection in Kassala, Sudan. PLoS neglected tropical diseases, PLoS Negl Trop Dis, doi:10.1371/journal.pntd.0009387
Breuer, Drysdale, Walker, Monitoring the emergence of resistance with sotrovimab in immunocompromised patients with COVID-19: LUNAR study, J Infect, doi:10.1016/j.jinf.2025.106510
Bronstein, Adler, Katash, Halutz, Levytskyi, Evolution of spike mutations following antibody treatment in two immunocompromised patients with persistent COVID-19 infection, J Med Virol, doi:10.1002/jmv.27445
Burnouf, Seghatchian, Ebola virus convalescent blood products: where we are now and where we may need to go, Transfus Apheresis Sci, doi:10.1016/j.transci.2014.10.003
Cao, Jian, Zhang, Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents, Cell Rep, doi:10.1016/j.celrep.2022.111845
Choi, Choudhary, Regan, Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host, N Engl J Med, doi:10.1056/NEJMc2031364
Choudhary, Chew, Deo, Emergence of SARS-CoV-2 escape mutations during Bamlanivimab therapy in a Phase II randomized clinical trial, Nature Microbiology, doi:10.1038/s41564-022-01254-1
Clark, Clark, Pan, SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms, Cell, doi:10.1016/j.cell.2021.03.027
Denkinger, Janssen, Schäkel, Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial, Nat Cancer, doi:10.1038/s43018-022-00503-w
Destras, Assaad, Bal, Bamlanivimab as monotherapy in two immunocompromised patients with COVID-19, Lancet Microbe, doi:10.1016/S2666-5247(21)00189-0
Destras, Bal, Simon, Lina, Josset, Sotrovimab drives SARS-CoV-2 Omicron variant evolution in immunocompromised patients, Lancet Microbe
Focosi, A Web Tool to Estimate Baseline Anti-Spike Monoclonal Antibody Efficacy Based on Regional Genomic Surveillance, Viruses, doi:10.3390/v15051048
Focosi, Alfonsi, Bernasconi, Is SARS-CoV-2 Spike Evolution Being Retargeted at the N-Terminal Domain?, Discov Med
Focosi, Casadevall, A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (Evusheld™) for COVID-19 prophylaxis and treatment, Viruses, doi:10.3390/v14091999
Focosi, Casadevall, Franchini, Sotrovimab: a Review of Its Efficacy against SARS-CoV-2 Variants, Viruses, doi:10.3390/v16020217
Focosi, Franchini, Casadevall, Maggi, An update on the anti-spike monoclonal antibody pipeline for SARS-CoV-2, Clin Microbiol Infect, doi:10.1016/j.cmi.2024.04.012
Focosi, Mcconnell, Sullivan, Casadevall, Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy, Drug Resist Updates, doi:10.1016/j.drup.2023.100991
Focosi, Novazzi, Baj, Sotrovimab-emergent resistance in SARS-CoV-2 Omicron: a series of three cases, J Clin Virol Plus, doi:10.1016/j.jcvp.2022.100097
Focosi, Novazzi, Genoni, None
Focosi, Spezia, Fixation and reversion of mutations in the receptor-binding domain of the SARS-CoV-2 spike protein: a 2020-2024 analysis, J Virus Eradic, doi:10.1016/j.jve.2024.100581
Focosi, Spezia, Gueli, The Era of the FLips: how Spike Mutations L455F and F456L (and A475V) Are Shaping SARS-CoV-2 Evolution, Viruses, doi:10.3390/v16010003
Focosi, Spezia, Subsequent Waves of Convergent Evolution in SARS-CoV-2 Genes and Proteins, Vaccines, doi:10.3390/vaccines12080887
Francica, Cai, Diallo, The SARS-CoV-2 Monoclonal Antibody AZD3152 Potently Neutralizes Historical and Emerging Variants and is Being Developed for the Prevention and Treatment of COVID-19 in High-Risk Individual
Gentile, Viceconte, Cuccurullo, Early combination of sotrovimab with nirmatrelvir/ritonavir or remdesivir is associated with low rate of persisting SARS CoV-2 infection in immunocompromised outpatients with mild-to-moderate COVID-19: a prospective single-centre study, doi:10.1080/07853890.2024.2439541
Gliga, Luebke, Killer, Rapid selection of sotrovimab escape variants in SARS-CoV-2 Omicron infected immunocompromised patients, Clinl Infect Dis, doi:10.1093/cid/ciac802
Gonzalez-Reiche, Alshammary, Schaefer, Intrahost Evolution and Forward Transmission of a Novel SARS-Cov-2 Omicron BA
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: a Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.0202JAMA
Gruber, Tucci, Rueca, Treatment-Emergent Cilgavimab Resistance Was Uncommon In Vaccinated Omicron BA.4/5 Outpatients, Biomolecules
Guigon, Faure, Lemaire, Emergence of Q493R mutation in SARS-CoV-2 spike protein during bamlanivimab/etesevimab treatment and resistance to viral clearance, J Infect, doi:10.1016/j.jinf.2021.08.033
Guo, Yu, Liu, Antigenic and Virological Characteristics of SARS-CoV-2 Variant BA.3.2, XFG, and NB, doi:10.1101/2025.04.30.651462
Gupta, Konnova, Smet, Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments, J Clin Invest
Haidar, Thomas, Loubet, Sipavibart for prevention of COVID-19 in immunocompromised persons (SUPERNOVA): a randomised, double-blind, phase 3 trial, Lancet Infect Dis
Halfmann, Minor, Haddock, La, Evolution of a globally unique SARS-CoV-2 Spike E484T monoclonal antibody escape mutation in a persistently infected, immunocompromised individual, Virus Evolution, doi:10.1093/ve/veac104
Hoffmann, Schrezenmeier, Desmarets, Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial, EBioMedicine, doi:10.1016/j.ebiom.2025.105613
Horby, Eberson, Peto, Sotrovimab versus usual care in patients admitted to hospital with COVID-19: a randomised, controlled, openlabel, platform trial (RECOVERY), Lancet Infect Dis
Huygens, Geurtsvankessel, Gharbharan, Clinical and Virological Outcome of Monoclonal Antibody Therapies Across SARS-CoV-2 Variants in 245 Immunocompromised Patients: a Multicenter Prospective Cohort Study, Clinl Infect Dis, doi:10.1093/cid/ciae026
Huygens, Munnink, Gharbharan, Koopmans, Rijnders, Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant, Clinl Infect Dis, doi:10.1093/cid/ciac601
Iriyama, Ichikawa, Tamura, Clinical and molecular landscape of prolonged SARS-CoV-2 infection with resistance to remdesivir in immunocompromised patients, PNAS nexus, doi:10.1093/pnasnexus/pgaf085
Iwasaki, Hashimoto, Takeuchi, Relapse of COVID-19 and Viral Evolution in a Patient With Good Syndrome: a Case Report, Cureus, doi:10.7759/cureus.52592
Jary, Marot, Faycal, Spike Gene Evolution and Immune Escape Mutations in Patients with Mild or Moderate Forms of COVID-19 and Treated with Monoclonal Antibodies Therapies, Viruses, doi:10.3390/v14020226
Jemielity, Wang, Chan, TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine, PLoS Pathogens, doi:10.1371/journal.ppat.1003232
Jensen, Luebke, Feldt, Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany, Lancet Regional Health, doi:10.1016/j.lanepe.2021.100164
Kijak, Ahani, Arbetter, Analysis of SARS-CoV-2 Emergent Variants Following AZD7442 (Tixagevimab/Cilgavimab) for Early Outpatient Treatment of COVID-19 (TACKLE Trial). Infectious diseases and therapy, Infectious Diseases and Therapy, doi:10.1007/s40121-023-00882-2
Leducq, Zafilaza, Fauchois, Spike Protein Genetic Evolution in Patients at High Risk of Severe Coronavirus Disease 2019 Treated by Monoclonal Antibodies, J Infect Dis, doi:10.1093/infdis/jiad523
Leducq, Zafilaza, Fauchois, Spike Protein Genetic Evolution in Patients at High Risk of Severe Coronavirus Disease 2019 Treated by Monoclonal Antibodies, J Infect Dis, doi:10.1093/infdis/jiad523
Li, Faraone, Hsu, Neutralization and Stability of JN.1-derived LB.1, KP.2.3, Subvariants. bioRxiv, doi:10.1101/2024.09.04.611219
Liu, Yu, Yang, Virological and antigenic characteristics of SARS-CoV-2 variants LF.7.2.1, NP.1, and LP.8.1, Lancet Infect Dis, doi:10.1101/2024.12.27.630350
Lohr, Niemann, Verheyen, Bamlanivimab treatment leads to rapid selection of immune escape variant carrying E484K mutation in a B.1.1.7 infected and immunosuppressed patient, Clinl Infect Dis, doi:10.1093/cid/ciab392
Marques, Graham-Wooten, Fitzgerald, SARS-CoV-2 evolution during prolonged infection in immunocompromised patients, mBio, doi:10.1128/mbio.00110-24
Mazzetti, Spezia, Capria, SARS-CoV-2 evolution during persistent infection in a CAR-T recipient shows an escape to both sotrovimab and T-cell responses, J Clin Virol Plus, doi:10.1016/j.jcvp.2023.100149
Montgomery, Hobbs, Padilla, Efficacy and safety of intramuscular administration of tixagevimab'cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a Phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00180-1
Palomino-Cabrera, Tejerina, Molero-Salinas, Frequent emergence of resistance mutations following complex intra-host genomic dynamics in SARS-CoV-2 patients receiving Sotrovimab, Antimicrob Agents Chemother, doi:10.1101/2023.03.01.530733
Paul, Choudhary, Heaps, Impact of SARS-CoV-2 Resistance to Antiviral Monoclonal Antibody Therapy on Neutralizing Antibody Response, Pathog Immun, doi:10.20411/pai.v9i2.718
Peiffer-Smadja, Bridier-Nahmias, Ferré, Emergence of E484K Mutation Following Bamlanivimab Monotherapy among High-Risk Patients Infected with the Alpha Variant of SARS-CoV-2, Viruses, doi:10.3390/v13081642
Phan, Zitzmann, Chew, Modeling the emergence of viral resistance for SARS-CoV-2 during treatment with an anti-spike monoclonal antibody, PLOS Pathogens, doi:10.1371/journal.ppat.1011680
Planas, Staropoli, Michel, Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86 lineages combining increased fitness and antibody evasion, bioRxiv, doi:10.1101/2023.11.20.567873
Planas, Staropoli, Planchais, Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP3.3 From Approved Monoclonal Antibodies, Pathog Immun, doi:10.20411/pai.v10i1.752
Pommeret, Colomba, Bigenwald, Bamlanivimab+ etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies, Ann Oncol, doi:10.1016/j.annonc.2021.07.015
Powers, Williams, Kreher, Neutralization of recent SARS-CoV-2 variants by genetically and structurally related mAbs of the pemivibart lineage, bioRxiv, doi:10.1101/2024.11.11.623127
Raglow, Surie, Chappell, SARS-CoV-2 shedding and evolution in patients who were immunocompromised during the omicron period: a multicentre, prospective analysis, Lancet Microbe, doi:10.1016/s2666-5247(23)00336-1
Ragonnet-Cronin, Nutalai, Huo, Generation of SARS-CoV-2 escape mutations by monoclonal antibody therapy, Nat Commun
Rockett, Basile, Maddocks, Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use, N Engl J Med, doi:10.1056/NEJMc2120219
Sabin, Richmond, Kenny, Emergence and onward transmission of a SARS-CoV-2 E484K variant among household contacts of a bamlanivimab-treated patient, Diagn Microbiol Infect Dis
Sanchez, Krantz, Yoke, Clinical outcomes and frequency of persistent infection among immunosuppressed patients treated with bebtelovimab for COVID-19 infection at an ambulatory cancer center. Transplant infectious disease, J Transplantation Society, doi:10.1111/tid.14223
Scherer, Babiker, Adelman, SARS-CoV-2 Evolution and Immune Escape in Immunocompromised Patients, N Engl J Med, doi:10.1056/NEJMc2202861
Schmidt, Li, Popejoy, Immunobridging for Pemivibart, a Monoclonal Antibody for Prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMc2404555
Schoefbaenker, Günther, Lorentzen, Characterisation of the antibody-mediated selective pressure driving intra-host evolution of SARS-CoV-2 in prolonged infection, PLOS Pathogens, doi:10.1371/journal.ppat.1012624
Simons, Ozer, Gambut, De novo emergence of SARS-CoV-2 spike mutations in immunosuppressed patients. Transplant infectious disease, J Transplantation Society, doi:10.1111/tid.13914
Subramanian, Schnell, Iulio, Resistance analysis following sotrovimab treatment in participants with COVID-19 during the Phase III COMET-ICE study, Future Virol, doi:10.2217/fvl-2023-0146
Tanino, Nishioka, Yamamoto, Emergence of SARS-CoV-2 with Dual-Drug Resistant Mutations During a Long-Term Infection in a Kidney Transplant Recipient, Infect Drug Resist, doi:10.2147/idr.s438915
Truffot, Andreani, Marechal, SARS-CoV-2 Variants in Immunocompromised Patient Given Antibody Monotherapy, Emerging Infectious Diseases
Uk, E virus via transfusion or transplantation
Uriu, Ito, Kosugi, Transmissibility, infectivity, and immune evasion of the SARS-CoV-2 BA.2.86 variant, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00575-3
Vellas, Bello, Influence of neutralizing monoclonal antibodies on the SARS-CoV-2 quasispecies in patients with COVID-19, Clin Microb Infect
Vellas, Kamar, Izopet, Resistance mutations in SARS-CoV-2 Omicron variant after tixagevimab-cilgavimab treatment, J Infect, doi:10.1016/j.jinf.2022.07.014
Vellas, Trémeaux, Bello, Resistance mutations in SARS-CoV-2 Omicron variant in patients treated with sotrovimab, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.05.002
Wang, Guo, Ho, Dd, Activity of Research-Grade Pemivibart against Recent SARS-CoV-2 JN.1 Sublineages, N Engl J Med, doi:10.1101/2024.08.12.607496
Wang, Guo, Mellis, Antibody evasiveness of SARS-CoV-2 subvariants KP.3.1.1 and XEC, Cell Rep, doi:10.1101/2024.11.17.624037
Wolfe, Cohen, Mahoney, Safety and Efficacy of Pemivibart, a Long-Acting Monoclonal Antibody, for Prevention of Symptomatic COVID-19: interim Results From the CANOPY Clinical Trial, Clinl Infect Dis, doi:10.1093/cid/ciaf265
Yang, Yu, Jian, Antigenicity and infectivity characterization of SARS-CoV-2 BA.2.86, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00573-X
Yang, Yu, Xu, Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure, Lancet Infect Dis, doi:10.1101/2023.11.13.566860
Yao, Ma, Lan, Guo, Liu, Neutralizing Activity and Viral Escape of Pemivibart by SARS-CoV-2 JN.1 sublineages, bioRxiv, doi:10.1101/2024.11.08.622746
Zafilaza, Bellet, Truffot, Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: a Multicentric Retrospective Study, Viruses
DOI record:
{
"DOI": "10.2147/idr.s540928",
"ISSN": [
"1178-6973"
],
"URL": "http://dx.doi.org/10.2147/IDR.S540928",
"author": [
{
"ORCID": "https://orcid.org/0000-0001-8811-195X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Focosi",
"given": "Daniele",
"sequence": "first"
},
{
"affiliation": [],
"family": "Franchini",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maggi",
"given": "Fabrizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casadevall",
"given": "Arturo",
"sequence": "additional"
}
],
"container-title": "Infection and Drug Resistance",
"container-title-short": "IDR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T10:30:13Z",
"timestamp": 1759314613000
},
"deposited": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T10:30:17Z",
"timestamp": 1759314617000
},
"indexed": {
"date-parts": [
[
2025,
10,
2
]
],
"date-time": "2025-10-02T00:43:11Z",
"timestamp": 1759365791129,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T00:00:00Z",
"timestamp": 1759276800000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/article/download/107500",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/article/download/107500",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "5207-5217",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2025,
10
]
]
},
"published-online": {
"date-parts": [
[
2025,
10
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"key": "ref1",
"unstructured": "US Food and Drug Administration. Emergency Use Authorization (EUA) for Bamlanivimab 700 mg and Etesevimab 1400 mg IV Administered Together Center for Drug Evaluation and Research (CDER) Review. 2021. Available from: https://www.fda.gov/media/146255/download. Accessed Sep 19, 2025."
},
{
"key": "ref2",
"unstructured": "European Medicines Agency. Assessment report Eli Lilly and Company Limited use of bamlanivimab and etesevimab for the treatment of COVID-19. 2021. Availablem from: https://www.ema.europa.eu/en/documents/referral/eli-lilly-company-limited-antibody-combination-bamlanivimab/etesevimab-covid19-article-53-procedure-assessment-report_en.pdf. Accessed Sep 19, 2025."
},
{
"DOI": "10.1056/NEJMoa2033130",
"doi-asserted-by": "crossref",
"key": "ref3",
"unstructured": "Activ-3/Tico Ly-CoV555 Study Group. A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(10):905–914. doi:10.1056/NEJMoa2033130"
},
{
"DOI": "10.1016/S2213-2600(22)00180-1",
"author": "Montgomery",
"doi-asserted-by": "publisher",
"first-page": "985",
"journal-title": "Lancet Respir Med",
"key": "ref4",
"volume": "10",
"year": "2022"
},
{
"author": "Horby",
"first-page": "1",
"journal-title": "Lancet Infect Dis",
"key": "ref5",
"volume": "2025",
"year": "2025"
},
{
"DOI": "10.1038/s43018-022-00503-w",
"author": "Denkinger",
"doi-asserted-by": "publisher",
"first-page": "96",
"journal-title": "Nat Cancer",
"key": "ref6",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1016/j.ebiom.2025.105613",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "105613",
"journal-title": "EBioMedicine",
"key": "ref7",
"volume": "113",
"year": "2025"
},
{
"DOI": "10.1093/infdis/jiad523",
"author": "Leducq",
"doi-asserted-by": "publisher",
"first-page": "1341",
"journal-title": "J Infect Dis",
"key": "ref8",
"volume": "229",
"year": "2024"
},
{
"DOI": "10.1038/s41564-022-01254-1",
"author": "Choudhary",
"doi-asserted-by": "publisher",
"first-page": "1906",
"journal-title": "Nature Microbiology",
"key": "ref9",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/j.xcrm.2022.100678",
"author": "Boucau",
"doi-asserted-by": "publisher",
"first-page": "100678",
"journal-title": "Cell Rep Med",
"key": "ref10",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.20411/pai.v9i2.718",
"author": "Paul",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Pathog Immun",
"key": "ref11",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.3201/eid2710.211509",
"author": "Truffot",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Emerging Infectious Diseases",
"key": "ref12",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab392",
"author": "Lohr",
"doi-asserted-by": "publisher",
"journal-title": "Clinl Infect Dis",
"key": "ref13",
"year": "2021"
},
{
"DOI": "10.1016/j.lanepe.2021.100164",
"author": "Jensen",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Lancet Regional Health",
"key": "ref14",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27445",
"author": "Bronstein",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "ref15",
"year": "2021"
},
{
"DOI": "10.1111/tid.13914",
"author": "Simons",
"doi-asserted-by": "publisher",
"first-page": "e13914",
"journal-title": "J Transplantation Society",
"key": "ref16",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.1016/j.diagmicrobio.2022.115656",
"author": "Sabin",
"doi-asserted-by": "crossref",
"first-page": "115656",
"journal-title": "Diagn Microbiol Infect Dis",
"key": "ref17",
"volume": "103",
"year": "2022"
},
{
"DOI": "10.1093/ve/veac104",
"author": "Halfmann",
"doi-asserted-by": "publisher",
"journal-title": "Virus Evolution",
"key": "ref18",
"year": "2022"
},
{
"DOI": "10.1016/S2666-5247(21)00189-0",
"author": "Destras",
"doi-asserted-by": "publisher",
"first-page": "e424",
"journal-title": "Lancet Microbe",
"key": "ref19",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1371/journal.ppat.1012624",
"author": "Schoefbaenker",
"doi-asserted-by": "publisher",
"first-page": "e1012624",
"journal-title": "PLOS Pathogens",
"key": "ref20",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1056/NEJMc2202861",
"author": "Scherer",
"doi-asserted-by": "publisher",
"first-page": "2436",
"journal-title": "N Engl J Med",
"key": "ref21",
"volume": "386",
"year": "2022"
},
{
"author": "Gupta",
"first-page": "1",
"journal-title": "J Clin Invest",
"key": "ref22",
"volume": "2023",
"year": "2023"
},
{
"DOI": "10.1177/13596535221097495",
"author": "Boussen",
"doi-asserted-by": "publisher",
"first-page": "13596535221097495",
"journal-title": "Antiviral Ther",
"key": "ref23",
"volume": "29",
"year": "2024"
},
{
"DOI": "10.3390/v14020226",
"author": "Jary",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref24",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/v13081642",
"author": "Peiffer-Smadja",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref25",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202JAMA",
"author": "Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "632",
"journal-title": "JAMA",
"key": "ref26",
"volume": "325",
"year": "2021"
},
{
"key": "ref27",
"unstructured": "Focosi D, Novazzi F, Genoni A, et al. 2021, Available from: https://www.researchsquare.com/article/rs-524959/v1. Accessed Sep 19, 2025."
},
{
"DOI": "10.1016/j.jinf.2021.08.033",
"author": "Guigon",
"doi-asserted-by": "publisher",
"first-page": "435",
"journal-title": "J Infect",
"key": "ref28",
"volume": "4453",
"year": "2021"
},
{
"author": "Vellas",
"first-page": "1",
"journal-title": "Clin Microb Infect",
"key": "ref29",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.3390/v16101542",
"author": "Zafilaza",
"doi-asserted-by": "crossref",
"first-page": "1542",
"journal-title": "Viruses",
"key": "ref30",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1016/j.annonc.2021.07.015",
"author": "Pommeret",
"doi-asserted-by": "publisher",
"journal-title": "Ann Oncol",
"key": "ref31",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2031364",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "2291",
"journal-title": "N Engl J Med",
"key": "ref32",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.03.027",
"author": "Clark",
"doi-asserted-by": "publisher",
"first-page": "2605",
"journal-title": "Cell",
"key": "ref33",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciae026",
"author": "Huygens",
"doi-asserted-by": "publisher",
"first-page": "1514",
"journal-title": "Clinl Infect Dis",
"key": "ref34",
"volume": "78",
"year": "2024"
},
{
"DOI": "10.7759/cureus.52592",
"author": "Iwasaki",
"doi-asserted-by": "publisher",
"first-page": "e52592",
"journal-title": "Cureus",
"key": "ref35",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1093/infdis/jiad523",
"author": "Leducq",
"doi-asserted-by": "publisher",
"first-page": "1341",
"journal-title": "J Infect Dis",
"key": "ref36",
"volume": "229",
"year": "2023"
},
{
"DOI": "10.1038/s41467-023-37826-w",
"author": "Ragonnet-Cronin",
"doi-asserted-by": "crossref",
"first-page": "3334",
"journal-title": "Nat Commun",
"key": "ref37",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2120219",
"author": "Rockett",
"doi-asserted-by": "publisher",
"first-page": "1477",
"journal-title": "N Engl J Med",
"key": "ref38",
"volume": "386",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.13854",
"author": "Birnie",
"doi-asserted-by": "publisher",
"first-page": "1104",
"journal-title": "JAMA",
"key": "ref39",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1016/j.jcvp.2022.100097",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "100097",
"journal-title": "J Clin Virol Plus",
"key": "ref40",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.05.002",
"author": "Vellas",
"doi-asserted-by": "publisher",
"first-page": "1297",
"journal-title": "Clin Microbiol Infect",
"key": "ref41",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac601",
"author": "Huygens",
"doi-asserted-by": "publisher",
"first-page": "e507",
"journal-title": "Clinl Infect Dis",
"key": "ref42",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2022.08.021",
"author": "Andrés",
"doi-asserted-by": "publisher",
"first-page": "240",
"journal-title": "Clin Microbiol Infect",
"key": "ref43",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.2217/fvl-2023-0146",
"author": "Subramanian",
"doi-asserted-by": "publisher",
"first-page": "975",
"journal-title": "Future Virol",
"key": "ref44",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1016/S2666-5247(22)00120-3",
"author": "Destras",
"doi-asserted-by": "crossref",
"first-page": "E559",
"journal-title": "Lancet Microbe",
"key": "ref45",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1101/2023.03.01.530733",
"author": "Palomino-Cabrera",
"doi-asserted-by": "publisher",
"first-page": "e0026623",
"journal-title": "Antimicrob Agents Chemother",
"key": "ref46",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2025.106510",
"author": "Breuer",
"doi-asserted-by": "publisher",
"first-page": "106510",
"journal-title": "J Infect",
"key": "ref47",
"volume": "91",
"year": "2025"
},
{
"DOI": "10.1093/pnasnexus/pgaf085",
"author": "Iriyama",
"doi-asserted-by": "publisher",
"first-page": "pgaf085",
"journal-title": "PNAS nexus",
"key": "ref48",
"volume": "4",
"year": "2025"
},
{
"DOI": "10.1128/mbio.00110-24",
"author": "Marques",
"doi-asserted-by": "publisher",
"first-page": "e0011024",
"journal-title": "mBio",
"key": "ref49",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.2147/idr.s438915",
"author": "Tanino",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Infect Drug Resist",
"key": "ref50",
"volume": "17",
"year": "2024"
},
{
"DOI": "10.1186/s12879-025-10740-w",
"author": "Bolis",
"doi-asserted-by": "publisher",
"first-page": "359",
"journal-title": "BMC Infect Dis",
"key": "ref51",
"volume": "25",
"year": "2025"
},
{
"DOI": "10.1016/j.jcvp.2023.100149",
"author": "Mazzetti",
"doi-asserted-by": "publisher",
"first-page": "100149",
"journal-title": "J Clin Virol Plus",
"key": "ref52",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac802",
"author": "Gliga",
"doi-asserted-by": "publisher",
"first-page": "408",
"journal-title": "Clinl Infect Dis",
"key": "ref53",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2022.07.014",
"author": "Vellas",
"doi-asserted-by": "publisher",
"first-page": "e162",
"journal-title": "J Infect",
"key": "ref54",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1007/s40121-023-00882-2",
"author": "Kijak",
"doi-asserted-by": "publisher",
"first-page": "2691",
"journal-title": "Infectious Diseases and Therapy",
"key": "ref55",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3390/biom13101538",
"author": "Gruber",
"doi-asserted-by": "crossref",
"first-page": "1538",
"journal-title": "Biomolecules",
"key": "ref56",
"volume": "13",
"year": "2023"
},
{
"key": "ref57",
"volume-title": "Intrahost Evolution and Forward Transmission of a Novel SARS-Cov-2 Omicron BA.1 Subvariant",
"year": "2022"
},
{
"DOI": "10.1111/tid.14223",
"author": "Sanchez",
"doi-asserted-by": "publisher",
"first-page": "e14223",
"journal-title": "J Transplantation Society",
"key": "ref58",
"volume": "26",
"year": "2024"
},
{
"DOI": "10.1371/journal.ppat.1011680",
"author": "Phan",
"doi-asserted-by": "publisher",
"first-page": "e1011680",
"journal-title": "PLOS Pathogens",
"key": "ref59",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1016/s2666-5247(23)00336-1",
"author": "Raglow",
"doi-asserted-by": "publisher",
"first-page": "e235",
"journal-title": "Lancet Microbe",
"key": "ref60",
"volume": "5",
"year": "2024"
},
{
"DOI": "10.1016/j.drup.2023.100991",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "100991",
"journal-title": "Drug Resist Updates",
"key": "ref61",
"volume": "71",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00575-3",
"author": "Uriu",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "ref62",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00573-X",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "e457",
"journal-title": "Lancet Infect Dis",
"key": "ref63",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofad500.1192",
"doi-asserted-by": "crossref",
"key": "ref64",
"unstructured": "Francica J, Cai Y, Diallo S, et al. The SARS-CoV-2 Monoclonal Antibody AZD3152 Potently Neutralizes Historical and Emerging Variants and is Being Developed for the Prevention and Treatment of COVID-19 in High-Risk Individual. 2023. Accessed from: https://congresspublication.com/fbkgfb?utm_term=Congress&utm_medium=Poster&utm_campaign=ID-Week-2023&utm_content=IDWeek-2023-AZD3152-PK-tox-poster&utm_source=82&utm_contentcategory=PDF#Congress. Accessed Sep 19, 2025."
},
{
"DOI": "10.1101/2023.11.13.566860",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "e70",
"journal-title": "Lancet Infect Dis",
"key": "ref65",
"volume": "24",
"year": "2023"
},
{
"key": "ref66",
"unstructured": "UK. Guidelines from the expert advisory committee on the Safety of Blood, Tissues and Organs (SaBTO) on measures to protect patients from acquiring hepatitis E virus via transfusion or transplantation. 2017. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/680297/Hepatitis_E_Guidelines.pdf. Accessed Sep 19, 2025."
},
{
"DOI": "10.1101/2023.11.20.567873",
"author": "Planas",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref67",
"year": "2023"
},
{
"DOI": "10.1101/2024.09.04.611219",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "611219",
"journal-title": "bioRxiv",
"key": "ref68",
"volume": "2024",
"year": "2024"
},
{
"DOI": "10.1101/2024.11.08.622746",
"author": "Yao",
"doi-asserted-by": "publisher",
"first-page": "622746",
"journal-title": "bioRxiv",
"key": "ref69",
"volume": "2024",
"year": "2024"
},
{
"DOI": "10.1101/2024.11.17.624037",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "115543",
"journal-title": "Cell Rep",
"key": "ref70",
"volume": "44",
"year": "2024"
},
{
"DOI": "10.1016/j.transci.2014.10.003",
"author": "Burnouf",
"doi-asserted-by": "publisher",
"first-page": "120",
"journal-title": "Transfus Apheresis Sci",
"key": "ref71",
"volume": "51",
"year": "2014"
},
{
"DOI": "10.1101/2024.08.12.607496",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1863",
"journal-title": "N Engl J Med",
"key": "ref72",
"volume": "391",
"year": "2024"
},
{
"DOI": "10.20411/pai.v10i1.752",
"author": "Planas",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Pathog Immun",
"key": "ref73",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1101/2024.11.11.623127",
"author": "Powers",
"doi-asserted-by": "publisher",
"first-page": "623127",
"journal-title": "bioRxiv",
"key": "ref74",
"volume": "2024",
"year": "2024"
},
{
"DOI": "10.1101/2024.12.27.630350",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "e128",
"journal-title": "Lancet Infect Dis",
"key": "ref75",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.1101/2025.04.30.651462",
"author": "Guo",
"doi-asserted-by": "publisher",
"first-page": "651462",
"journal-title": "bioRxiv",
"key": "ref76",
"volume": "2025",
"year": "2025"
},
{
"DOI": "10.3390/vaccines12080887",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "887",
"journal-title": "Vaccines",
"key": "ref77",
"volume": "12",
"year": "2024"
},
{
"DOI": "10.3390/v16010003",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Viruses",
"key": "ref78",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1016/j.jve.2024.100581",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "100581",
"journal-title": "J Virus Eradic",
"key": "ref79",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1371/journal.ppat.1003232",
"author": "Jemielity",
"doi-asserted-by": "publisher",
"first-page": "e1003232",
"journal-title": "PLoS Pathogens",
"key": "ref80",
"volume": "9",
"year": "2013"
},
{
"DOI": "10.3390/v15051048",
"author": "Focosi",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref81",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2404555",
"author": "Schmidt",
"doi-asserted-by": "publisher",
"first-page": "1860",
"journal-title": "N Engl J Med",
"key": "ref82",
"volume": "391",
"year": "2024"
},
{
"DOI": "10.1101/2024.12.16.628620",
"author": "Bernasconi",
"doi-asserted-by": "publisher",
"first-page": "12",
"journal-title": "bioRxiv",
"key": "ref83",
"volume": "2024",
"year": "2024"
},
{
"DOI": "10.3390/v14091999",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "1999",
"journal-title": "Viruses",
"key": "ref84",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/v16020217",
"author": "Focosi",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref85",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1016/j.cmi.2024.04.012",
"author": "Focosi",
"doi-asserted-by": "publisher",
"first-page": "999",
"journal-title": "Clin Microbiol Infect",
"key": "ref86",
"volume": "30",
"year": "2024"
},
{
"author": "Haidar",
"first-page": "1",
"journal-title": "Lancet Infect Dis",
"key": "ref87",
"volume": "2025",
"year": "2025"
},
{
"DOI": "10.1093/cid/ciaf265",
"author": "Wolfe",
"doi-asserted-by": "publisher",
"journal-title": "Clinl Infect Dis",
"key": "ref88",
"year": "2025"
},
{
"DOI": "10.1016/j.celrep.2022.111845",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "111845",
"journal-title": "Cell Rep",
"key": "ref89",
"volume": "41",
"year": "2022"
},
{
"DOI": "10.1371/journal.pntd.0009387",
"author": "Bower",
"doi-asserted-by": "publisher",
"first-page": "e0009387",
"journal-title": "PLoS Negl Trop Dis",
"key": "ref90",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2023.8592",
"author": "Ahiskali",
"doi-asserted-by": "publisher",
"first-page": "e238592",
"journal-title": "JAMA Netw Open",
"key": "ref91",
"volume": "6",
"year": "2023"
},
{
"author": "Focosi",
"first-page": "1",
"journal-title": "Discov Med",
"key": "ref92",
"volume": "2025",
"year": "2025"
},
{
"DOI": "10.1080/07853890.2024.2439541",
"author": "Gentile",
"doi-asserted-by": "publisher",
"first-page": "2439541",
"journal-title": "Ann Med",
"key": "ref93",
"volume": "57",
"year": "2025"
}
],
"reference-count": 93,
"references-count": 93,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/the-emergence-of-escape-mutations-in-covid-19-following-anti-spike-mon-peer-reviewed-fulltext-article-IDR"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The Emergence of Escape Mutations in COVID-19 Following Anti-Spike Monoclonal Antibody Treatment: How Do We Tackle It?",
"type": "journal-article",
"volume": "Volume 18"
}
focosi5
