Rapid Selection of Sotrovimab Escape Variants in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron-Infected Immunocompromised Patients
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac802, Oct 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
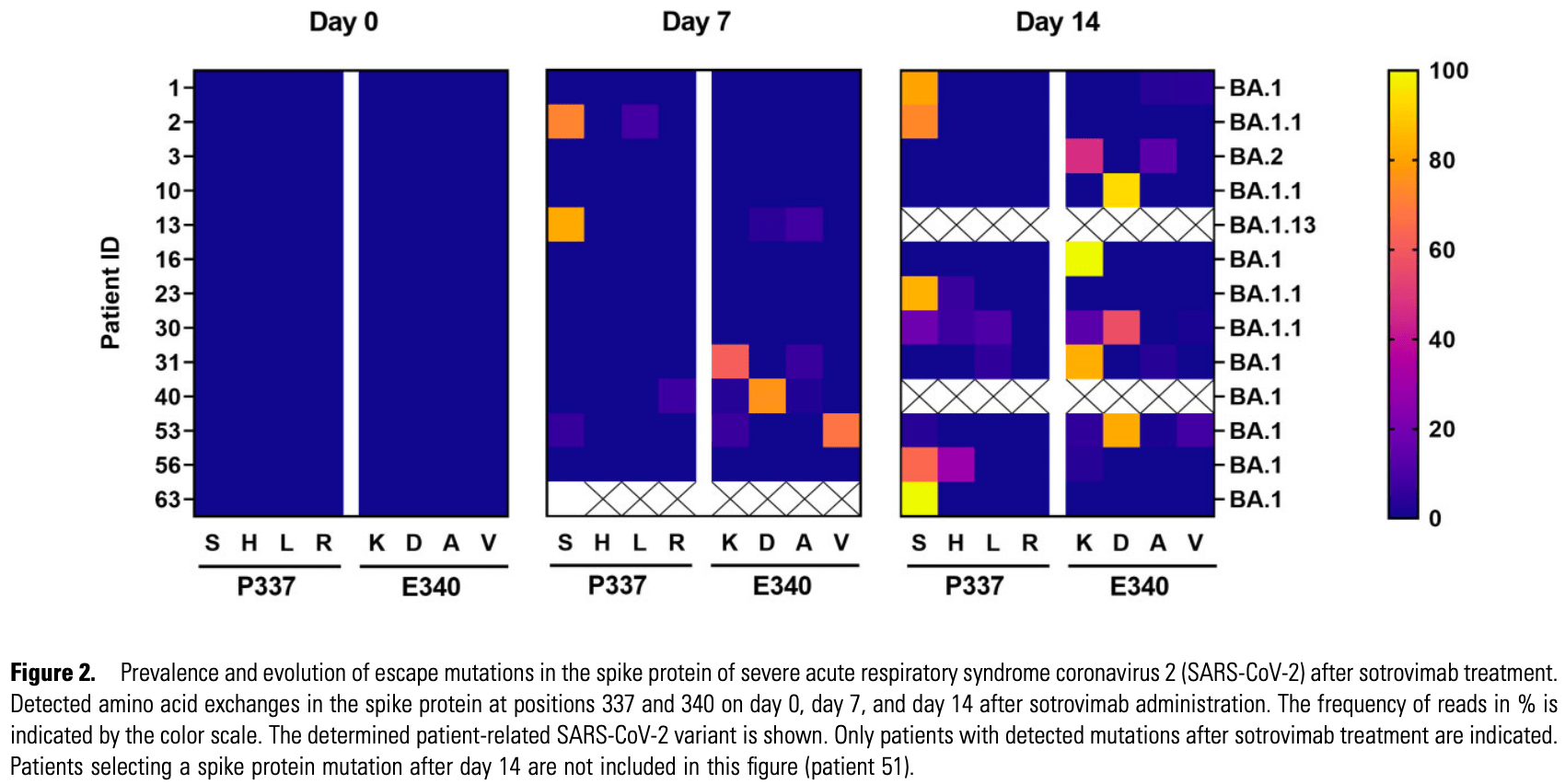
Prospective analysis of 57 COVID-19 patients receiving sotrovimab, showing rapid creation of escape mutations within immunodeficient patients. Combined treatment with remdesivir reduced the creation of escape variants.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Gliga et al., 3 Oct 2022, prospective, Germany, peer-reviewed, 21 authors, study period 20 January, 2022 - 25 February, 2022.
Contact: nadine.luebke@med.uniduesseldorf.de.
Rapid Selection of Sotrovimab Escape Variants in Severe Acute Respiratory Syndrome Coronavirus 2 Omicron-Infected Immunocompromised Patients
Clinical Infectious Diseases, doi:10.1093/cid/ciac802
Background. Monoclonal antibodies (mAbs) that target severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are predominantly less effective against Omicron variants. Immunocompromised patients often experience prolonged viral shedding, resulting in an increased risk of viral escape. Methods. In an observational, prospective cohort, 57 patients infected with Omicron variants who received sotrovimab alone or in combination with remdesivir were followed. The study end points were a decrease in SARS-CoV-2 RNA <10 6 copies/mL in nasopharyngeal swabs at day 21 and the emergence of escape mutations at days 7, 14, and 21 after sotrovimab administration. All SARS-CoV-2 samples were analyzed using whole-genome sequencing. Individual variants within the quasispecies were subsequently quantified and further characterized using a pseudovirus neutralization assay. Results. The majority of patients (43 of 57, 75.4%) were immunodeficient, predominantly due to immunosuppression after organ transplantation or hematologic malignancies. Infections by Omicron/BA.1 comprised 82.5%, while 17.5% were infected by Omicron/ BA.2. Twenty-one days after sotrovimab administration, 12 of 43 (27.9%) immunodeficient patients had prolonged viral shedding compared with 1 of 14 (7.1%) immunocompetent patients (P = .011). Viral spike protein mutations, some specific for Omicron (e.g., P337S and/or E340D/V), emerged in 14 of 43 (32.6%) immunodeficient patients, substantially reducing sensitivity to sotrovimab in a pseudovirus neutralization assay. Combination therapy with remdesivir significantly reduced emergence of escape variants. Conclusions. Immunocompromised patients face a considerable risk of prolonged viral shedding and emergence of escape mutations after early therapy with sotrovimab. These findings underscore the importance of careful monitoring and the need for dedicated clinical trials in this patient population.
Supplementary Data Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
References
Abdullah, Myers, Basu, Decreased severity of disease during the first global Omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa, Int J Infect Dis, doi:10.1016/j.ijid.2021.12.357
Author Contributions, for conceptualization and supervised the study
Bruel, Hadjadj, Maes, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med, doi:10.1038/s41591-022-01792-5
Choi, Choudhary, Regan, Persistence and evolution of SARS-CoV-2 in an immunocompromised host, N Engl J Med, doi:10.1056/NEJMc2031364
Corey, Beyrer, Cohen, Michael, Bedford et al., SARS-CoV-2 variants in patients with immunosuppression, N Engl J Med, doi:10.1056/NEJMsb2104756
Destras, Bal, Simon, Lina, Josset, Sotrovimab drives SARS-CoV-2 Omicron variant evolution in immunocompromised patients, Lancet Microbe, doi:10.1016/s2666-5247(22)00120-3
Gandhi, Klein, Robertson, De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report, Nat Commun, doi:10.1038/s41467-022-29104-y
Group, Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00751-9
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Hoffmann, Kruger, Schulz, The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic, Cell, doi:10.1016/j.cell.2021.12.032
Jefferson, Spencer, Brassey, Heneghan, Viral cultures for coronavirus disease 2019 infectivity assessment: a systematic review, Clin Infect Dis, doi:10.1093/cid/ciaa1764
Jensen, Luebke, Feldt, Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2021.100164
Kreuzberger, Hirsch, Chai, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013825.pub2
Leung, Chorlton, Tyson, COVID-19 in an immunocompromised host: persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: a case report, Int J Infect Dis, doi:10.1016/j.ijid.2021.10.045
Martin-Blondel, Marcelin, Soulie, Outcome of very high-risk patients treated by sotrovimab for mild-to-moderate COVID-19 Omicron, a prospective cohort study (the ANRS 0003S COCOPREV study), J Infect, doi:10.1016/j.jinf.2022.04.010
Peiffer-Smadja, Bridier-Nahmias, Ferre, Emergence of E484K mutation following bamlanivimab monotherapy among high-risk patients infected with the Alpha variant of SARS-CoV-2, Viruses, doi:10.3390/v13081642
Rockett, Basile, Maddocks, Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use, N Engl J Med, doi:10.1056/NEJMc2120219
Sanjuán, Domingo-Calap, Genetic diversity and evolution of viral populations, Encyclopedia Virol, doi:10.1016/B978-0-12-809633-8.20958-8
Singh, Sharma, Lee, Yadav, SARS-CoV-2: recent variants and clinical efficacy of antibody-based therapy, Front Cell Infect Microbiol, doi:10.3389/fcimb.2022.839170
Solera, Arbol, Alshahrani, Impact of vaccination and early monoclonal antibody therapy on COVID-19 outcomes in organ transplant recipients during the Omicron wave, Clin Infect Dis, doi:10.1093/cid/ciac324
Takashita, Kinoshita, Yamayoshi, Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Tallarita, Giardina, Novazzi, Spread of multiple SARS-CoV-2 lineages April-August 2020 anticipated the second pandemic wave in Lombardy (Italy), Pediatr Allergy Immunol, doi:10.1111/pai.13641
Truong, Ryutov, Pandey, Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series, EBioMedicine, doi:10.1016/j.ebiom.2021.103355
Vellas, Bello, Debard, Influence of treatment with neutralizing monoclonal antibodies on the SARS-CoV-2 nasopharyngeal load and quasispecies, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.09.008
Vellas, Tremeaux, Bello, Resistance mutations in SARS-CoV-2 Omicron variant in patients treated with sotrovimab, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.05.002
Yamasoba, Kosugi, Kimura, Neutralisation sensitivity of SARS-CoV-2 Omicron subvariants to therapeutic monoclonal antibodies, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00365-6
DOI record:
{
"DOI": "10.1093/cid/ciac802",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac802",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Monoclonal antibodies (mAbs) that target severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are predominantly less effective against Omicron variants. Immunocompromised patients often experience prolonged viral shedding, resulting in an increased risk of viral escape.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In an observational, prospective cohort, 57 patients infected with Omicron variants who received sotrovimab alone or in combination with remdesivir were followed. The study end points were a decrease in SARS-CoV-2 RNA &lt;106 copies/mL in nasopharyngeal swabs at day 21 and the emergence of escape mutations at days 7, 14, and 21 after sotrovimab administration. All SARS-CoV-2 samples were analyzed using whole-genome sequencing. Individual variants within the quasispecies were subsequently quantified and further characterized using a pseudovirus neutralization assay.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The majority of patients (43 of 57, 75.4%) were immunodeficient, predominantly due to immunosuppression after organ transplantation or hematologic malignancies. Infections by Omicron/BA.1 comprised 82.5%, while 17.5% were infected by Omicron/BA.2. Twenty-one days after sotrovimab administration, 12 of 43 (27.9%) immunodeficient patients had prolonged viral shedding compared with 1 of 14 (7.1%) immunocompetent patients (P = .011). Viral spike protein mutations, some specific for Omicron (e.g., P337S and/or E340D/V), emerged in 14 of 43 (32.6%) immunodeficient patients, substantially reducing sensitivity to sotrovimab in a pseudovirus neutralization assay. Combination therapy with remdesivir significantly reduced emergence of escape variants.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Immunocompromised patients face a considerable risk of prolonged viral shedding and emergence of escape mutations after early therapy with sotrovimab. These findings underscore the importance of careful monitoring and the need for dedicated clinical trials in this patient population.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
},
{
"name": "Institute of Virology, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf , Düsseldorf , Germany"
}
],
"family": "Gliga",
"given": "Smaranda",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-3820-7915",
"affiliation": [
{
"name": "Institute of Virology, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf , Düsseldorf , Germany"
}
],
"authenticated-orcid": false,
"family": "Lübke",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Killer",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Virology, Faculty of Medicine and University Hospital Cologne, University of Cologne , Cologne , Germany"
}
],
"family": "Gruell",
"given": "Henning",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Virology, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf , Düsseldorf , Germany"
}
],
"family": "Walker",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Medical Microbiology and Hospital Hygiene, Medical Faculty, Heinrich-Heine-University Düsseldorf , Düsseldorf , Germany"
}
],
"family": "Dilthey",
"given": "Alexander T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Immunology and Genetics , Kaiserslautern , Germany"
}
],
"family": "Thielen",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Lohr",
"given": "Carolin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Flaßhove",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Krieg",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Pereira",
"given": "Joanna Ventura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Seraphin",
"given": "Tobias Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Zaufel",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Immunology and Genetics , Kaiserslautern , Germany"
}
],
"family": "Däumer",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Orth",
"given": "Hans-Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Feldt",
"given": "Torsten",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Bode",
"given": "Johannes G",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Virology, Faculty of Medicine and University Hospital Cologne, University of Cologne , Cologne , Germany"
},
{
"name": "Center for Molecular Medicine Cologne, University of Cologne , Cologne , Germany"
}
],
"family": "Klein",
"given": "Florian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Virology, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf , Düsseldorf , Germany"
}
],
"family": "Timm",
"given": "Jörg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Luedde",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany"
}
],
"family": "Jensen",
"given": "Björn-Erik Ole",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T20:31:10Z",
"timestamp": 1664569870000
},
"deposited": {
"date-parts": [
[
2022,
11,
2
]
],
"date-time": "2022-11-02T17:38:38Z",
"timestamp": 1667410718000
},
"funder": [
{
"award": [
"01KX2021"
],
"name": "COVIM"
},
{
"DOI": "10.13039/501100002347",
"doi-asserted-by": "crossref",
"name": "Federal Ministry of Education and Research"
},
{
"DOI": "10.13039/501100000780",
"award": [
"101046016"
],
"doi-asserted-by": "crossref",
"name": "European Commission"
},
{
"name": "Ministry of Culture and Science of the State of North Rhine-Westphalia"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
20
]
],
"date-time": "2022-12-20T17:18:27Z",
"timestamp": 1671556707887
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2022,
10,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
3
]
],
"date-time": "2022-10-03T00:00:00Z",
"timestamp": 1664755200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac802/46752524/ciac802.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac802/46752524/ciac802.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
10,
3
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
3
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1002/14651858.CD013825.pub2",
"article-title": "SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19",
"author": "Kreuzberger",
"doi-asserted-by": "publisher",
"journal-title": "Cochrane Database Syst Rev",
"key": "2022110217382301200_ciac802-B1",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.12.032",
"article-title": "The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "Cell",
"key": "2022110217382301200_ciac802-B2",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "2022110217382301200_ciac802-B3",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "1236",
"journal-title": "JAMA",
"key": "2022110217382301200_ciac802-B4",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00365-6",
"article-title": "Neutralisation sensitivity of SARS-CoV-2 Omicron subvariants to therapeutic monoclonal antibodies",
"author": "Yamasoba",
"doi-asserted-by": "publisher",
"first-page": "942",
"journal-title": "Lancet Infect Dis",
"key": "2022110217382301200_ciac802-B5",
"volume": "22",
"year": "2022"
},
{
"key": "2022110217382301200_ciac802-B6"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"author": "Group AC-TfIwC-S",
"doi-asserted-by": "publisher",
"first-page": "622",
"journal-title": "Lancet Infect Dis",
"key": "2022110217382301200_ciac802-B7",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.04.010",
"article-title": "Outcome of very high-risk patients treated by sotrovimab for mild-to-moderate COVID-19 Omicron, a prospective cohort study (the ANRS 0003S COCOPREV study)",
"author": "Martin-Blondel",
"doi-asserted-by": "publisher",
"first-page": "e101",
"journal-title": "J Infect",
"key": "2022110217382301200_ciac802-B8",
"volume": "84",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac324",
"article-title": "Impact of vaccination and early monoclonal antibody therapy on COVID-19 outcomes in organ transplant recipients during the Omicron wave",
"author": "Solera",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "2022110217382301200_ciac802-B9"
},
{
"DOI": "10.1016/j.lanepe.2021.100164",
"article-title": "Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany",
"author": "Jensen",
"doi-asserted-by": "publisher",
"first-page": "100164",
"journal-title": "Lancet Reg Health Eur",
"key": "2022110217382301200_ciac802-B10",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/s2666-5247(22)00120-3",
"article-title": "Sotrovimab drives SARS-CoV-2 Omicron variant evolution in immunocompromised patients",
"author": "Destras",
"doi-asserted-by": "publisher",
"first-page": "e559",
"journal-title": "Lancet Microbe",
"key": "2022110217382301200_ciac802-B11",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.05.002",
"article-title": "Resistance mutations in SARS-CoV-2 Omicron variant in patients treated with sotrovimab",
"author": "Vellas",
"doi-asserted-by": "publisher",
"first-page": "1297",
"journal-title": "Clin Microbiol Infect",
"key": "2022110217382301200_ciac802-B12",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciaa1764",
"article-title": "Viral cultures for coronavirus disease 2019 infectivity assessment: a systematic review",
"author": "Jefferson",
"doi-asserted-by": "publisher",
"first-page": "e3884",
"journal-title": "Clin Infect Dis",
"key": "2022110217382301200_ciac802-B13",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1111/pai.13641",
"article-title": "Spread of multiple SARS-CoV-2 lineages April-August 2020 anticipated the second pandemic wave in Lombardy (Italy)",
"author": "Tallarita",
"doi-asserted-by": "publisher",
"first-page": "89",
"issue": "(Suppl 27)",
"journal-title": "Pediatr Allergy Immunol",
"key": "2022110217382301200_ciac802-B14",
"volume": "33",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2021.12.357",
"article-title": "Decreased severity of disease during the first global Omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa",
"author": "Abdullah",
"doi-asserted-by": "publisher",
"first-page": "38",
"journal-title": "Int J Infect Dis",
"key": "2022110217382301200_ciac802-B15",
"volume": "116",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "publisher",
"first-page": "1297",
"journal-title": "Nat Med",
"key": "2022110217382301200_ciac802-B16",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "publisher",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "2022110217382301200_ciac802-B17",
"volume": "386",
"year": "2022"
},
{
"key": "2022110217382301200_ciac802-B18"
},
{
"DOI": "10.1056/NEJMc2031364",
"article-title": "Persistence and evolution of SARS-CoV-2 in an immunocompromised host",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "2291",
"journal-title": "N Engl J Med",
"key": "2022110217382301200_ciac802-B19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMsb2104756",
"article-title": "SARS-CoV-2 variants in patients with immunosuppression",
"author": "Corey",
"doi-asserted-by": "publisher",
"first-page": "562",
"journal-title": "N Engl J Med",
"key": "2022110217382301200_ciac802-B20",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103355",
"article-title": "Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series",
"author": "Truong",
"doi-asserted-by": "publisher",
"first-page": "103355",
"journal-title": "EBioMedicine",
"key": "2022110217382301200_ciac802-B21",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-29104-y",
"article-title": "De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report",
"author": "Gandhi",
"doi-asserted-by": "publisher",
"first-page": "1547",
"journal-title": "Nat Commun",
"key": "2022110217382301200_ciac802-B22",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2021.10.045",
"article-title": "COVID-19 in an immunocompromised host: persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: a case report",
"author": "Leung",
"doi-asserted-by": "publisher",
"first-page": "178",
"journal-title": "Int J Infect Dis",
"key": "2022110217382301200_ciac802-B23",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.09.008",
"article-title": "Influence of treatment with neutralizing monoclonal antibodies on the SARS-CoV-2 nasopharyngeal load and quasispecies",
"author": "Vellas",
"doi-asserted-by": "publisher",
"first-page": "139.e5",
"journal-title": "Clin Microbiol Infect",
"key": "2022110217382301200_ciac802-B24",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2120219",
"article-title": "Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use",
"author": "Rockett",
"doi-asserted-by": "publisher",
"first-page": "1477",
"journal-title": "N Engl J Med",
"key": "2022110217382301200_ciac802-B25",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3389/fcimb.2022.839170",
"article-title": "SARS-CoV-2: recent variants and clinical efficacy of antibody-based therapy",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "839170",
"journal-title": "Front Cell Infect Microbiol",
"key": "2022110217382301200_ciac802-B26",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/v13081642",
"article-title": "Emergence of E484K mutation following bamlanivimab monotherapy among high-risk patients infected with the Alpha variant of SARS-CoV-2",
"author": "Peiffer-Smadja",
"doi-asserted-by": "publisher",
"first-page": "1642",
"journal-title": "Viruses",
"key": "2022110217382301200_ciac802-B27",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/B978-0-12-809633-8.20958-8",
"article-title": "Genetic diversity and evolution of viral populations",
"author": "Sanjuán",
"doi-asserted-by": "publisher",
"first-page": "53",
"journal-title": "Encyclopedia Virol",
"key": "2022110217382301200_ciac802-B28",
"year": "2021"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac802/6746719"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Rapid Selection of Sotrovimab Escape Variants in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron-Infected Immunocompromised Patients",
"type": "journal-article"
}
