Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(21)00751-9, ACTIV-3/TICO, NCT04501978, Dec 2021
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
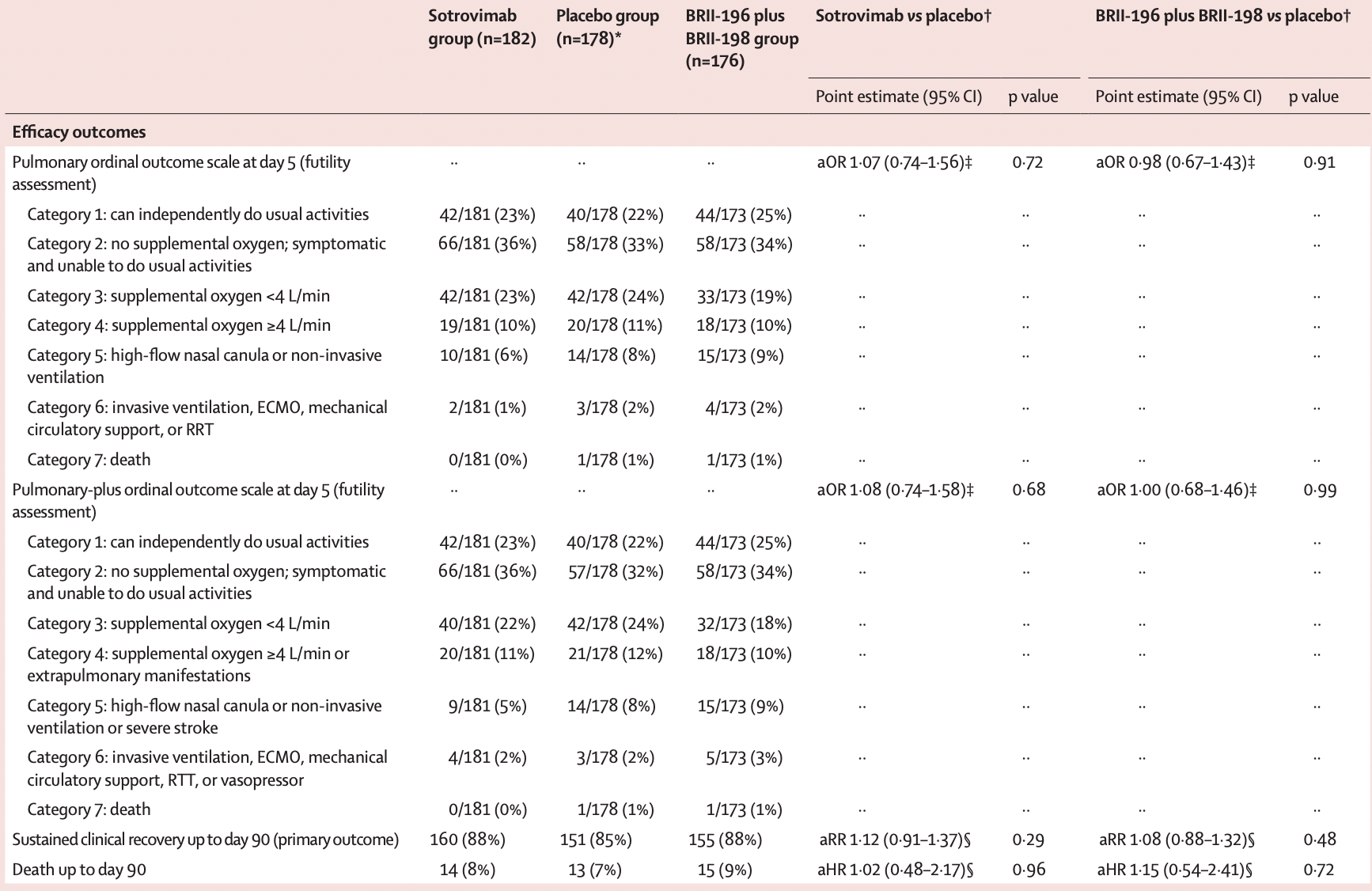
RCT with 182 sotrovimab patients, 176 BRII-196+BRII-198 patients, and 178 control patients, median 8 days from symptom onset, showing no significant differences and terminated early due to futility. Long-term results are reported in Mourad et al.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.12-4, BA.4, BA.55, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.16, and no efficacy for BA.27, XBB, XBB.1.5, ХВВ.1.9.18, XBB.1.16, BQ.1.1.45, and CL.16. US EUA has been revoked.
Study covers sotrovimab and amubarvimab.
|
risk of death, 2.0% higher, RR 1.02, p = 0.96, treatment 14 of 182 (7.7%), control 13 of 178 (7.3%), adjusted per study, day 90.
|
|
risk of no recovery, 10.7% lower, RR 0.89, p = 0.29, treatment 22 of 182 (12.1%), control 27 of 178 (15.2%), NNT 32, adjusted per study, inverted to make RR<1 favor treatment, day 90, primary outcome.
|
|
risk of no recovery, 7.4% lower, RR 0.93, p = 0.69, treatment 181, control 178, adjusted per study, inverted to make RR<1 favor treatment, pulmonary-plus ordinal outcome @day 5, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Mourad et al., Long-term outcomes of passive immunotherapy for COVID-19: a pooled analysis of a large multinational platform randomized clinical trial, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2025.02.002.
2.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
3.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
4.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
5.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
6.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Self et al., 23 Dec 2021, Double Blind Randomized Controlled Trial, multiple countries, peer-reviewed, 67 authors, study period 16 December, 2020 - 1 March, 2021, average treatment delay 8.0 days, trial NCT04501978 (history) (ACTIV-3/TICO).
Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(21)00751-9
Background We aimed to assess the efficacy and safety of two neutralising monoclonal antibody therapies (sotrovimab [Vir Biotechnology and GlaxoSmithKline] and BRII-196 plus BRII-198 [Brii Biosciences]) for adults admitted to hospital for COVID-19 (hereafter referred to as hospitalised) with COVID-19. Methods In this multinational, double-blind, randomised, placebo-controlled, clinical trial (Therapeutics for Inpatients with COVID-19 [TICO]), adults (aged ≥18 years) hospitalised with COVID-19 at 43 hospitals in the USA, Denmark, Switzerland, and Poland were recruited. Patients were eligible if they had laboratory-confirmed SARS-CoV-2 infection and COVID-19 symptoms for up to 12 days. Using a web-based application, participants were randomly assigned (2:1:2:1), stratified by trial site pharmacy, to sotrovimab 500 mg, matching placebo for sotrovimab, BRII-196 1000 mg plus BRII-198 1000 mg, or matching placebo for BRII-196 plus BRII-198, in addition to standard of care. Each study product was administered as a single dose given intravenously over 60 min. The concurrent placebo groups were pooled for analyses. The primary outcome was time to sustained clinical recovery, defined as discharge from the hospital to home and remaining at home for 14 consecutive days, up to day 90 after randomisation. Interim futility analyses were based on two seven-category ordinal outcome scales on day 5 that measured pulmonary status and extrapulmonary complications of COVID-19. The safety outcome was a composite of death, serious adverse events, incident organ failure, and serious coinfection up to day 90 after randomisation. Efficacy and safety outcomes were assessed in the modified intention-to-treat population, defined as all patients randomly assigned to treatment who started the study infusion. This study is registered with ClinicalTrials.gov, NCT04501978.
Contributors Author and collaborator contributions, including responsibility for decision to submit the manuscript, drafting of the initial manuscript, study conceptualisation, investigation, data curation, formal analysis, study supervision, and review and editing of the manuscript, are provided in the appendix (pp 4-11). JDN and GG directly accessed and verified the underlying study data. JDN had access to all the study data and had final responsibility for the decision to submit the paper for publication.
Declaration of interests
References
Arvin, Fink, Schmid, A perspective on potential antibody-dependent enhancement of SARS-CoV-2, Nature
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19-final report, N Engl J Med
Biosciences, Brii Biosciences announces positive data from the phase 3 ACTIV-2 trial evaluating combination BRII-196 and BRII-198 in non-hospitalized COVID-19 patients
Biosciences, Durham, Usa (d Margolis, Zhu, Phd, None
Cao, None
Cathcart, Havenar-Daughton, Lempp, The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2, bioRxiv, doi:10.1101/2021.03.09.434607
Datta-Mannan, Mechanisms influencing the pharmacokinetics and disposition of monoclonal antibodies and peptides, Drug Metab Dispos
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med
Glaxosmithkline, Vir Biotechnology and GSK announce VIR-7831 reduces hospitalisation and risk of death in early treatment of adults with COVID-19
Globalnewswire, Vir Biotechnology and GSK announce VIR-7831 reduces hospitalization and risk of death in early treatment of adults with COVID-19
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Gupta, Gonzalez-Rojas, Juarez, Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Horby, Mafham, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial medRxiv, doi:10.1101/2021.06.15.21258542
Ju, Zhang, Ge, Human neutralizing antibodies elicited by SARS-CoV-2 infection, Nature
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with COVID-19, N Engl J Med
Kan, Infectious Diseases Section
Kirby Institute, Hang, Prof, Polizzotto, Vincent, Hospital
Lee, Wheatley, Kent, Dekosky, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies, Nat Microbiol
Lilly, Lilly's bamlanivimab and etesevimab together reduced hospitalizations and death in phase 3 trial for early COVID-19
Murray, Babiker, Baker, Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: therapeutics for inpatients with COVID-19 (TICO/ACTIV-3), Clinical Trials
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature
Schuler Cf 4th, Gherasim, Shea, Accurate point-of-care serology tests for COVID-19, PLoS One
Self, Semler, Leither, Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial, JAMA
Tico Bamlanivimab, Group, Lundgren, Grund, Clinical and virological response to a neutralizing monoclonal antibody for hospitalized patients with COVID-19, medRxiv, doi:10.1101/2021.07.19.21260559
Tuccori, Ferraro, Convertino, Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline, MAbs
Usa, Osei, None
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Yang, Liu, Yu, Wu, Reichert et al., COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19, Antib Ther
DOI record:
{
"DOI": "10.1016/s1473-3099(21)00751-9",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/s1473-3099(21)00751-9",
"alternative-id": [
"S1473309921007519"
],
"author": [
{
"affiliation": [],
"family": "Self",
"given": "Wesley H.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sandkovsky",
"given": "Uriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reilly",
"given": "Cavan S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vock",
"given": "David M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gottlieb",
"given": "Robert L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mack",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Golden",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dishner",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vekstein",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ko",
"given": "Emily R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Der",
"given": "Tatyana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Franzone",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almasri",
"given": "Eyad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fayed",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Filbin",
"given": "Michael R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hibbert",
"given": "Kathryn A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rice",
"given": "Todd W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casey",
"given": "Jonathan D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayanga",
"given": "J. Awori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badhwar",
"given": "Vinay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leshnower",
"given": "Bradley G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharifpour",
"given": "Milad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knowlton",
"given": "Kirk U.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peltan",
"given": "Ithan D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bakowska",
"given": "Elizieta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowalska",
"given": "Justyna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bowdish",
"given": "Michael E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sturek",
"given": "Jeffrey M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rogers",
"given": "Angela J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Files",
"given": "D. Clark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mosier",
"given": "Jarrod M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gong",
"given": "Michelle N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douin",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hite",
"given": "R. Duncan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trautner",
"given": "Barbara W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jain",
"given": "Mamta K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gardner",
"given": "Edward M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Akram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Jens-Ulrik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matthay",
"given": "Michael A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Samuel M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Higgs",
"given": "Elizabeth S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pett",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weintrob",
"given": "Amy C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Christina C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murrary",
"given": "Daniel D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Günthard",
"given": "Huldrych F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moquete",
"given": "Ellen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grandits",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engen",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grund",
"given": "Birgit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Shweta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Huyen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osei",
"given": "Suzette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Margolis",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Qing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Polizzotto",
"given": "Mark N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babiker",
"given": "Abdel G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davey",
"given": "Victoria J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kan",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "B. Taylor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gelijns",
"given": "Annetine C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neaton",
"given": "James D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lane",
"given": "H. Clifford",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jundgren",
"given": "Jens D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tierney",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barrett",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herpin",
"given": "Betsey R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smolskis",
"given": "Mary C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Voge",
"given": "Susan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McNay",
"given": "Laura A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cahill",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crew",
"given": "Page",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kirchoff",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sardana",
"given": "Ratna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raim",
"given": "Sharon Segal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiu",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hensley",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorenzo",
"given": "Josua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mock",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaw-Saliba",
"given": "Katy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zuckerman",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adam",
"given": "Stacey J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Currier",
"given": "Judy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Read",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hughes",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amos",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carlsen",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carter",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davis",
"given": "Bionca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Denning",
"given": "Eileen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DuChene",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harrison",
"given": "Merrie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaiser",
"given": "Payton",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koopmeiners",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meger",
"given": "Sue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murray",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quan",
"given": "Kien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quan",
"given": "Siu Fun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walski",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wentworth",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moskowitz",
"given": "Alan J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bagiella",
"given": "Emilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O'Sullivan",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marks",
"given": "Mary E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Accardi",
"given": "Evan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinzel",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bedoya",
"given": "Gabriela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Lopa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Overbey",
"given": "Jessica R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Padillia",
"given": "Maria L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Milerva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gillinov",
"given": "Marc A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Marissa A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taddei-Peters",
"given": "Wendy C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fenton",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berhe",
"given": "Mezgebe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haley",
"given": "Clinton",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bettacchi",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duhaime",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ryan",
"given": "Madison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burris",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Felecia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villa",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Want",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robert",
"given": "Raven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coleman",
"given": "Tanquinisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clariday",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baker",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hurutado-Rodriguez",
"given": "Marian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iram",
"given": "Nazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fresnedo",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davis",
"given": "Allyson",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leonard",
"given": "Kiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramierez",
"given": "Noelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thammavong",
"given": "Jon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duque",
"given": "Krizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turner",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fisher",
"given": "Tammy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Dianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ransom",
"given": "Desirae",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lusk",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Killian",
"given": "Aaron",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palacious",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solis",
"given": "Edilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jerrow",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watts",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Whitacre",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cothran",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Peter K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barkauskas",
"given": "Christina E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dreyer",
"given": "Grace R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Witte",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mosaly",
"given": "Nilima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourad",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holland",
"given": "Thomas L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lane",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouffler",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGowan",
"given": "Lauren M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Motta",
"given": "Marry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tipton",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stallings",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stout",
"given": "Gennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McLendon-Arvik",
"given": "Beth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hollister",
"given": "Beth A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giangiacomo",
"given": "Dana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Sunil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pappers",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCarthy",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krupica",
"given": "Troy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sarwari",
"given": "Arif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reece",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fornaresio",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Glaze",
"given": "Chad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Raquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Preamble",
"given": "Katarina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sutton",
"given": "Lisa Giblin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buterbaugh",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bartolo",
"given": "Elizabeth Berry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bunner",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bender",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baio",
"given": "Kim T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McBride",
"given": "Mary K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fielding",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathewson",
"given": "Sonya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Porte",
"given": "Kristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maton",
"given": "Missy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponder",
"given": "Chari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haley",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spainhour",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rogers",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tyler",
"given": "Derrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wald-Dickler",
"given": "Noah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutcheon",
"given": "Douglass",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Towfighi",
"given": "Amytis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "May M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewis",
"given": "Meghan R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spellberg",
"given": "Brad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sher",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Aniket",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olds",
"given": "Anna P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Justino",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lozano",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leong",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodina",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Possemato",
"given": "Tammie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Escobar",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiu",
"given": "Charlene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weissman",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barros",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Enfield",
"given": "Kyle B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kadl",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Green",
"given": "China J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simon",
"given": "Rachel M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thornton",
"given": "Kara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parrino",
"given": "Patrick E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spindel",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bansal",
"given": "Aditya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baumgarten",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hand",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vonderhaar",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nossaman",
"given": "Bobby",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laudun",
"given": "Sylvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ames",
"given": "DeAnna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Broussard",
"given": "Shane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernandez",
"given": "Nilmo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Isaac",
"given": "Geralyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dinh",
"given": "Huan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Yiling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tran",
"given": "Sonny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McDaniel",
"given": "Hunter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crovetto",
"given": "Nicolle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schelle",
"given": "Beth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McLean",
"given": "Sherry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rothbaum",
"given": "Howard R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alvarez",
"given": "Michael S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalan",
"given": "Shivam P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Germann",
"given": "Heather H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hendershot",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maroney",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herring",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cook",
"given": "Sharri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paul",
"given": "Pam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madathil",
"given": "Ronson J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rabin",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levine",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saharia",
"given": "Kapil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tabatabai",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lau",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gammie",
"given": "James S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peguero",
"given": "Maya-Loren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKernan",
"given": "Kimberley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Audette",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fleischmann",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akbari",
"given": "Freshta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Maia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Myounghee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chi",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salehi",
"given": "Hanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pariser",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Phuong Tran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gee",
"given": "Adrienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vincent",
"given": "Shelika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zuckerman",
"given": "Richard A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iribarne",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Metzler",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shipman",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caccia",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Haley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Newton",
"given": "Crystallee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parr",
"given": "Doug",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodriguez",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bokhart",
"given": "Gordon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eichman",
"given": "Sharon M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "North",
"given": "Crystal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oldmixon",
"given": "Cathryn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ringwood",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fitzgerald",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morin",
"given": "Haley D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muzikansky",
"given": "Ariela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morse",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brower",
"given": "Roy G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reineck",
"given": "Lora A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aggarwal",
"given": "Neil R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bienstock",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hou",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Steingrub",
"given": "Jay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tidswell",
"given": "Mark A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kozikowski",
"given": "Lori-Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kardos",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DeSouza",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thornton-Thompson",
"given": "Sherell",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talmor",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shapiro",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banner-Goodspeed",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyle",
"given": "Katherine L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayes",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Alan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galbraith",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nandi",
"given": "Utsav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peacock",
"given": "Rebekah K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parry",
"given": "Blair Alden",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Margolin",
"given": "Justin D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brait",
"given": "Kelsey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beakes",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kangelaris",
"given": "Kirsten N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yee",
"given": "Kimberly J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ashktorab",
"given": "Kimia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jauregui",
"given": "Alejandra E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhuo",
"given": "Hanjing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hendey",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hubel",
"given": "Kinsley A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hughes",
"given": "Alyssa R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Rebekah L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilson",
"given": "Jennifer G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vojnik",
"given": "Rosemary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roque",
"given": "Jonasel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "George W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Steven Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beutler",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agarwal",
"given": "Trisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vargas",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moss",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baduashvili",
"given": "Amiran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chauhan",
"given": "Lakshmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Finck",
"given": "Lani L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Howell",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hyzy",
"given": "Robert C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Pauline K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nelson",
"given": "Kristine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McSparron",
"given": "Jake I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Co",
"given": "Ivan N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Bonnie R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jia",
"given": "Shijing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sullins",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanna",
"given": "Sinan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olbrich",
"given": "Norman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richardson",
"given": "Lynne D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nair",
"given": "Rahul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Offor",
"given": "Obiageli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amosu",
"given": "Omowunmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tzehaie",
"given": "Hiwet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terndrup",
"given": "Thomas E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiedemann",
"given": "Herbert P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duggal",
"given": "Abhijit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thiruchelvam",
"given": "Nirosshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ashok",
"given": "Kiran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "King",
"given": "Alexander H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehkri",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hudock",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiran",
"given": "Simra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "More",
"given": "Harshada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roads",
"given": "Tammy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinkovic",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kennedy",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Bryce H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hough",
"given": "Catherine L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krol",
"given": "Olivia F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinjal",
"given": "Mistry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mills",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McDougal",
"given": "Madeline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deshmukh",
"given": "Rupali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torbati",
"given": "Sam S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matusov",
"given": "Yuri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choe",
"given": "June",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hindoyan",
"given": "Niree A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jackman",
"given": "Susan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayoumi",
"given": "Emad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wynter",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caudill",
"given": "Antonina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pascual",
"given": "Ethan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clapham",
"given": "Gregg J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herrera",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ojukwu",
"given": "Cristabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehdikhani",
"given": "Shaunt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O'Mahony",
"given": "D. Shane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nyatsatsang",
"given": "Sonam T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilson",
"given": "David M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wallick",
"given": "Julie A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Chadwick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibbs",
"given": "Keven W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores",
"given": "Lori S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LaRose",
"given": "Mary E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landreth",
"given": "Leigha D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morris",
"given": "Peter E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sturgill",
"given": "Jamie L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cassity",
"given": "Evan P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhar",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montgomery-Yates",
"given": "Ashley A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pasha",
"given": "Sara N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayer",
"given": "Kirby P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bissel",
"given": "Brittany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bledsoe",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanspa",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leither",
"given": "Lindsey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Armbruster",
"given": "Brent P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montgomery",
"given": "Quinn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Applegate",
"given": "Darrin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Naresh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fergus",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Serezlic",
"given": "Erna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imel",
"given": "Karah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palmer",
"given": "Ghazal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Webb",
"given": "Brandon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aston",
"given": "Valerie T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Jakea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gray",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hays",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roth",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Popielski",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivasplata",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turner",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vjecha",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petersen",
"given": "Tianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamel",
"given": "Dena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hansen",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lucas",
"given": "Claudia Sanchez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DellaValle",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonzales",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scott",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wyles",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douglas",
"given": "Ivor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haukoos",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamis",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Caitlin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baker",
"given": "Jason V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frosch",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldsmith",
"given": "Rachael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jibrell",
"given": "Hodan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lo",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klaphake",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mackedanz",
"given": "Shari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngo",
"given": "Linh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia-Myers",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Markowitz",
"given": "Norman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pastor",
"given": "Erika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramesh",
"given": "Mayur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brar",
"given": "Indira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivers",
"given": "Emanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Princy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Menna",
"given": "Maximiliano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biswas",
"given": "Kousick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harrington",
"given": "Cristin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delp",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pandit",
"given": "Lavannya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hines-Munson",
"given": "Casey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dillon",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Want",
"given": "Yiqun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lichtenberger",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baracco",
"given": "Gio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramos",
"given": "Carol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bjork",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sueiro",
"given": "Melyssa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tien",
"given": "Phyllis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freasier",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buck",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nekach",
"given": "Hafida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nagy-Agren",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vasudeva",
"given": "Shikha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ochalek",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roller",
"given": "Brentin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Chinh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mikail",
"given": "Amani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raben",
"given": "Dorthe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Tomas O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aagaard",
"given": "Bitten",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Charlotte B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krapp",
"given": "Katharina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nykjær",
"given": "Bente Rosdahl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanne",
"given": "Katja Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grevsen",
"given": "Anne Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joensen",
"given": "Zillah Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruun",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bojesen",
"given": "Ane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woldbye",
"given": "Frederik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Normand",
"given": "Nick E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esmann",
"given": "Frederik V.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clausen",
"given": "Clara Lundetoft",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hovmand",
"given": "Nichlas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pedersen",
"given": "Karen Brorup",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thorlacius-Ussing",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tinggaard",
"given": "Michaela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Høgsberg",
"given": "Dorthe S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rastoder",
"given": "Ema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamstrup",
"given": "Thobias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bergsøe",
"given": "Christina Marisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Østergaard",
"given": "Lars",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stærke",
"given": "Nina Breinholt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johansen",
"given": "Isik S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knudtzen",
"given": "Fredrikke C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larsen",
"given": "Lykke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hertz",
"given": "Mathias A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fabricius",
"given": "Thilde",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Helleberg",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gerstoft",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Tomas Østergaard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindegaard",
"given": "Birgitte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pedersen",
"given": "Thomas Ingemann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Røge",
"given": "Birgit Thorup",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Løfberg",
"given": "Sandra Valborg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hansen",
"given": "Thomas Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Ariella Denize",
"sequence": "additional"
},
{
"affiliation": [],
"family": "von Huth",
"given": "Sebastian Leicht",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Henrik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thisted",
"given": "Rikke Krog",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Podlekareva",
"given": "Daria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnsen",
"given": "Stine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andreassen",
"given": "Helle Frost",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pedersen",
"given": "Lars",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindnér",
"given": "Cecilia Ebba Clara Ellinor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiese",
"given": "Lothar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knudsen",
"given": "Lene Surland",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nytofte",
"given": "Nikolaj Julian Skrøder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Havmøller",
"given": "Signe Ravn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paredes",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Exposito",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández-Cruz",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arribas",
"given": "Jose R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estrada",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Horcajada",
"given": "Juan P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burgos",
"given": "Joaquin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morales-Rull",
"given": "Jose Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Braun",
"given": "Dominique L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "West",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "M'Rabeth-Bensalah",
"given": "Khadija",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eichinger",
"given": "Mareile L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grüttner-Durmaz",
"given": "Manuela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grube",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zink",
"given": "Veronika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Horban",
"given": "Andrzej",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bednarska",
"given": "Agnieszka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jurek",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fätkenheuer",
"given": "Gerd",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malinm",
"given": "Jakob J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matthews",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kelleher",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabrera",
"given": "Gesalit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carey",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hough",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Virachit",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Young",
"given": "Barnaby E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chia",
"given": "Po Ying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Tau Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Ray J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lye",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ong",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puah",
"given": "Ser Hon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yeo",
"given": "Tsin Wen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diong",
"given": "Shiau Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ongko",
"given": "Juwinda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hudson",
"given": "Fleur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parmar",
"given": "Mahesh KB",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodman",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badrock",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gregory",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Touloumi",
"given": "Giota",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pantaz",
"given": "Nikos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gioukari",
"given": "Vicky",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lutaakome",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kityo",
"given": "Cissy M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mugerwa",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiweewa",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osinusi",
"given": "Anu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tipple",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Willis",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peppercorn",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watson",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alexander",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mogalian",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Leo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ding",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Girardet",
"given": "Jean-Luc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Ji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Zhi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adams",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albert",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balde",
"given": "Abby",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baracz",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baseler",
"given": "Beth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Becker",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bielica",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Billouin-Frazier",
"given": "Shere",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cash",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhary",
"given": "Jay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dolney",
"given": "Suzanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dixon",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eyler",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frye",
"given": "Leanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galcik",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gertz",
"given": "Jensen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giebeig",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gulati",
"given": "Neelam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hankinson",
"given": "Liz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hissey",
"given": "Debbie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hogarty",
"given": "Debi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hohn",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holley",
"given": "H Preston",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoopengardner",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huber",
"given": "Lynda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jankelevich",
"given": "Shirley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krauss",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lake",
"given": "Eileen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Linton",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacDonald",
"given": "Leah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manandhar",
"given": "Meryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spinelli-Nadzam",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oluremi",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Proffitt",
"given": "Calvin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rudzinski",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sandrus",
"given": "Jen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaffhauser",
"given": "Marylu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schechner",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suders",
"given": "Connie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gerry",
"given": "Norman P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Kenneth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solomon",
"given": "Courtney",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kubernac",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rashid",
"given": "Marium",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Bhakti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kubernac",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murphy",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoover",
"given": "Marie L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DuChateau",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flosi",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Les",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Treagus",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wenner",
"given": "Christine",
"sequence": "additional"
}
],
"container-title": [
"The Lancet Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
24
]
],
"date-time": "2021-12-24T00:22:11Z",
"timestamp": 1640305331000
},
"deposited": {
"date-parts": [
[
2021,
12,
24
]
],
"date-time": "2021-12-24T00:22:34Z",
"timestamp": 1640305354000
},
"funder": [
{
"DOI": "10.13039/100000050",
"doi-asserted-by": "publisher",
"name": "National Heart Lung and Blood Institute"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
24
]
],
"date-time": "2021-12-24T00:41:05Z",
"timestamp": 1640306465261
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1473-3099"
}
],
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309921007519?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309921007519?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib1",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with COVID-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib3",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib4",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(21)00751-9_bib5",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1177/17407745211049829",
"article-title": "Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: therapeutics for inpatients with COVID-19 (TICO/ACTIV-3)",
"author": "Murray",
"doi-asserted-by": "crossref",
"journal-title": "Clinical Trials",
"key": "10.1016/S1473-3099(21)00751-9_bib11",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A neutralizing monoclonal antibody for hospitalized patients with COVID-19",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib12",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1080/19420862.2020.1854149",
"article-title": "Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline",
"author": "Tuccori",
"doi-asserted-by": "crossref",
"journal-title": "MAbs",
"key": "10.1016/S1473-3099(21)00751-9_bib13",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2349-y",
"article-title": "Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody",
"author": "Pinto",
"doi-asserted-by": "crossref",
"first-page": "290",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(21)00751-9_bib14",
"volume": "583",
"year": "2020"
},
{
"article-title": "The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2",
"author": "Cathcart",
"journal-title": "bioRxiv",
"key": "10.1016/S1473-3099(21)00751-9_bib15",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2380-z",
"article-title": "Human neutralizing antibodies elicited by SARS-CoV-2 infection",
"author": "Ju",
"doi-asserted-by": "crossref",
"first-page": "115",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(21)00751-9_bib16",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1124/dmd.119.086488",
"article-title": "Mechanisms influencing the pharmacokinetics and disposition of monoclonal antibodies and peptides",
"author": "Datta-Mannan",
"doi-asserted-by": "crossref",
"first-page": "1100",
"journal-title": "Drug Metab Dispos",
"key": "10.1016/S1473-3099(21)00751-9_bib17",
"volume": "47",
"year": "2019"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(21)00751-9_bib18",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22240",
"article-title": "Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial",
"author": "Self",
"doi-asserted-by": "crossref",
"first-page": "2165",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(21)00751-9_bib19",
"volume": "324",
"year": "2020"
},
{
"article-title": "COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19",
"author": "Yang",
"first-page": "205",
"journal-title": "Antib Ther",
"key": "10.1016/S1473-3099(21)00751-9_bib20",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib21",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00751-9_bib22",
"volume": "385",
"year": "2021"
},
{
"article-title": "Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"journal-title": "medRxiv",
"key": "10.1016/S1473-3099(21)00751-9_bib23",
"year": "2021"
},
{
"article-title": "Clinical and virological response to a neutralizing monoclonal antibody for hospitalized patients with COVID-19",
"author": "Lundgren",
"journal-title": "medRxiv",
"key": "10.1016/S1473-3099(21)00751-9_bib27",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0248729",
"article-title": "Accurate point-of-care serology tests for COVID-19",
"author": "Schuler",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S1473-3099(21)00751-9_bib28",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2538-8",
"article-title": "A perspective on potential antibody-dependent enhancement of SARS-CoV-2",
"author": "Arvin",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(21)00751-9_bib30",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-00789-5",
"article-title": "Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "1185",
"journal-title": "Nat Microbiol",
"key": "10.1016/S1473-3099(21)00751-9_bib31",
"volume": "5",
"year": "2020"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"score": 1,
"short-container-title": [
"The Lancet Infectious Diseases"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": [
"Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial"
],
"type": "journal-article"
}
