Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial
et al., eBioMedicine, doi:10.1016/j.ebiom.2025.105613, COVIC-19, NCT05271929, Feb 2025
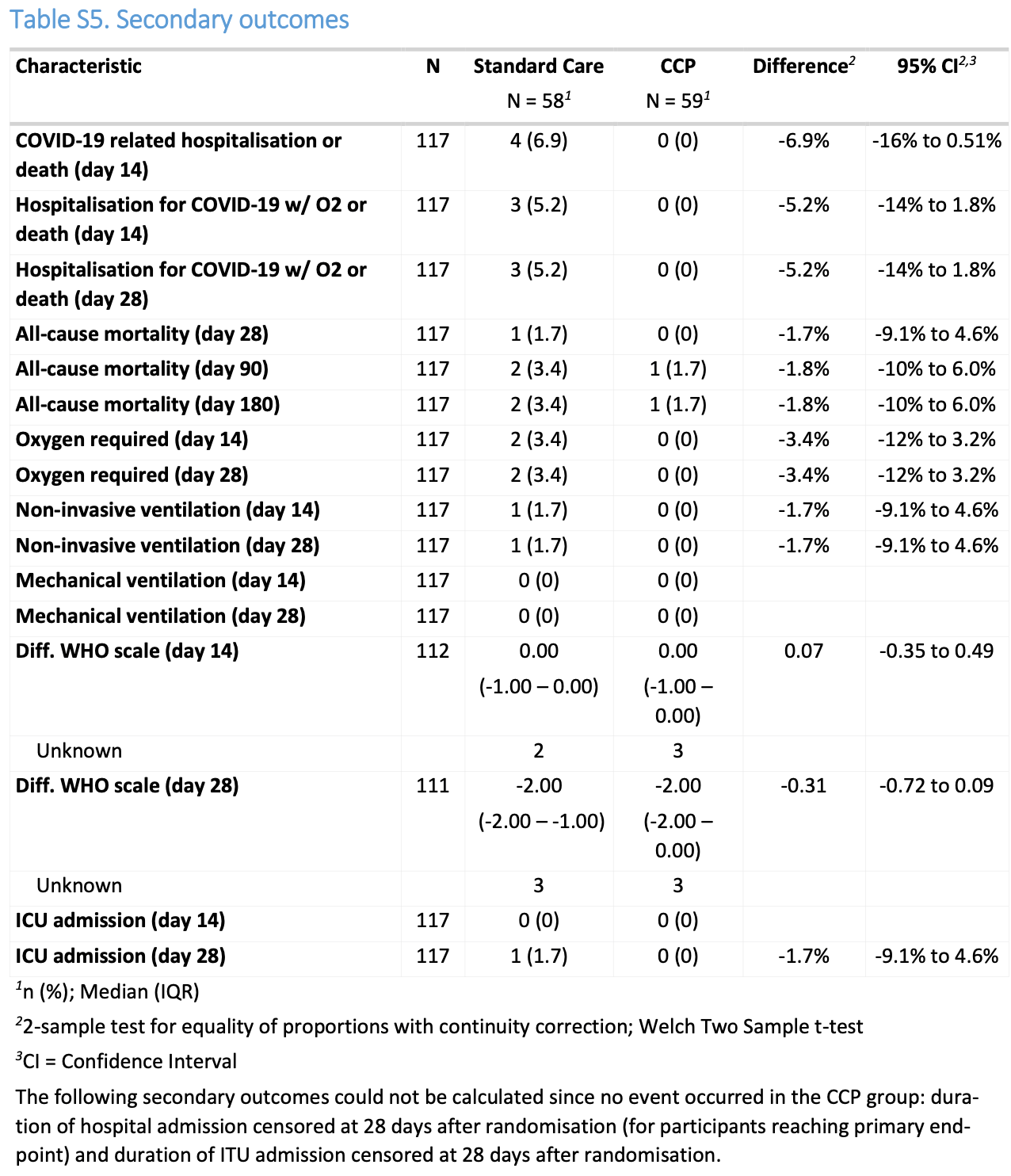
RCT 117 immunocompromised patients with mild COVID-19 showing lower hospitalization or death with early administration of very high-titre COVID-19 convalescent plasma (CCP). The trial was terminated early due to declining enrollment.
|
risk of death, 50.8% lower, RR 0.49, p = 0.62, treatment 1 of 59 (1.7%), control 2 of 58 (3.4%), NNT 57, day 180.
|
|
risk of death/hospitalization, 91.0% lower, RR 0.09, p = 0.03, treatment 0 of 59 (0.0%), control 5 of 58 (8.6%), NNT 12, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
|
risk of death/hospitalization, 89.0% lower, RR 0.11, p = 0.06, treatment 0 of 59 (0.0%), control 4 of 58 (6.9%), NNT 14, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
|
risk of ICU admission, 66.9% lower, RR 0.33, p = 0.50, treatment 0 of 59 (0.0%), control 1 of 58 (1.7%), NNT 58, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hoffmann et al., 27 Feb 2025, Randomized Controlled Trial, multiple countries, peer-reviewed, median age 57.0, 33 authors, study period 11 April, 2022 - 27 November, 2023, average treatment delay 4.0 days, trial NCT05271929 (history) (COVIC-19).
Contact: h.schrezenmeier@blutspende.de.
Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial
eBioMedicine, doi:10.1016/j.ebiom.2025.105613
Background COVID-19 convalescent plasma (CCP) is a treatment option for COVID-19. This study investigated the safety and efficacy of early, very high-titre CCP in immunocompromised individuals with mild COVID-19. Methods This randomised, controlled, open-label trial assessed CCP in immunocompromised patients (n = 120) with mild COVID-19 in 10 clinical trial centres across Germany, France, and the Netherlands. Patients were randomised 1:1 to receive either standard of care (SoC) alone (SoC group) or SoC and 2 units of CCP. Most patients (89.7%) had received ≥3 SARS-CoV-2 vaccinations. The primary endpoint was hospitalisation for progressive COVID-19 symptoms or death by day 28 after randomisation, analysed on a modified intention-to-treat basis (117 patients). The safety analysis included the full analysis set. The trial is registered with EudraCT 2021-006621-22, and ClinicalTrials.gov, NCT05271929. Findings Between April 11, 2022 and November 27, 2023, 120 patients were enrolled. Patients in the CCP group received a median of 559 ml CCP from convalescent, vaccinated donors with very high levels of SARS-CoV-2 antibodies (median 81,810 IU/ml) at a median 4 days after symptom onset. The primary outcome occurred in 5/58 patients (8.6%) in the SoC group and in 0/59 patients (0%) in the CCP group, difference -8.6% (95% confidence interval of difference -19% to -0.80%; p-value 0.027; Fisher's exact test). The course of SARS-CoV-2 antibodies in the patients
Contributors Conceptualisaton: LJE, DJR, DB, CV, PT, MD, and HS generated the study design and wrote the study protocol. Project administration: SH, SK, CV, GB, MD, PT, ET, BR, and HS coordinated the trial. Supervision: HS, AD, and ET, and BR: Principal Investigators in Germany, France, and Netherlands. Formal analysis: MD trial statistician, developed the statistical analysis plan and performed the statistical analysis. The first three authors and the last author wrote the first draft of the manuscript. All the authors contributed to subsequent drafts. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. All authors contributed to interpretation of data, manuscript writing and approved the manuscript.
Data sharing statement Anonymised data will be available upon request for independent review panel-approved research proposals with a signed data sharing agreement. The data will be available after approval and data sharing agreement is in place until 3 years after publication. The data can be obtained by request to maxime.desmarets@univ-fcomte.fr.
Declaration of interests PT is an employee of Établissement Français du Sang, the blood establishment responsible for blood collection, qualification and supply in France. HS, SH, SK, HH, MS are, and ESe was an employee of the German Red Cross Blood Transfusion Service Baden-Württemberg-Hessen (or its affiliates), the establishment responsible for blood collection,..
References
Abbasi, Researchers tie severe immunosuppression to chronic COVID-19 and virus variants, JAMA
Alemany, Millat-Martinez, Corbacho-Monne, High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial, Lancet Respir Med
Bar, Shaw, Choi, A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia, J Clin Invest
Biernat, Kolasinska, Kwiatkowski, Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID-19, Viruses
Cele, Gazy, Jackson, Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma, Nature
Denkinger, Janssen, Schakel, Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial, Nat Cancer
Desmarets, Hoffmann, Vauchy, Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19 (COVIC-19): protocol for a randomised, openlabel trial, BMJ Open
Dewolf, Laracy, Perales, Kamboj, Van Den Brink et al., SARS-CoV-2 in immunocompromised individuals, Immunity
Estcourt, Turgeon, Mcquilten, Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial, JAMA
Focosi, Franchini, Pirofski, COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors, Viruses
Franchini, Cruciani, Casadevall, Safety of COVID-19 convalescent plasma: a definitive systematic review and metaanalysis of randomized controlled trials, Transfusion
Gharbharan, Jordans, Zwaginga, Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial, Clin Microbiol Infect
Goyal, Duke, Cardozo-Ojeda, Schiffer, Modeling explains prolonged SARS-CoV-2 nasal shedding relative to lung shedding in remdesivir-treated rhesus macaques, iScience
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Hueso, Godron, Lanoy, Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis, Leukemia
Iannizzi, Chai, Piechotta, Convalescent plasma for people with COVID-19: a living systematic review, Cochrane Database Syst Rev
Jahrsdorfer, Proffen, Scholz, BNT162b2 booster vaccination elicits cross-reactive immunity against SARS-CoV-2 variants B.1.1.529 and B.1.617.2 in convalescents of all ages, Front Immunol
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med
Kemp, Collier, Datir, SARS-CoV-2 evolution during treatment of chronic infection, Nature
Korley, Durkalski-Mauldin, Yeatts, Early convalescent plasma for high-risk outpatients with Covid-19, N Engl J Med
Korper, Gruner, Zickler, One-year follow-up of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients, J Clin Invest
Körper, Weiss, Zickler, Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19, J Clin Invest
Lacombe, Hueso, Porcher, Use of covid-19 convalescent plasma to treat patients admitted to hospital for covid-19 with or without underlying immunodeficiency: open label, randomised clinical trial, BMJ Med
Libster, Perez, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
Markov, Ghafari, Beer, The evolution of SARS-CoV-2, Nat Rev Microbiol
Mccarthy, Rennick, Nambulli, Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape, Science
Millat-Martinez, Gharbharan, Alemany, Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients, Nat Commun
Newcombe, Interval estimation for the difference between independent proportions: comparison of eleven methods, Stat Med
Osmanodja, Ronicke, Budde, Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients, J Clin Med
Pinana, Vazquez, Heras, Omicron SARS-CoV-2 infection management and outcomes in patients with hematologic disease and recipients of cell therapy, Front Oncol
Planas, Staropoli, Planchais, Escape of SARS-CoV-2 variants KP.1.1, LB.1, and KP3.3 from approved monoclonal antibodies, Pathog Immun
Rincon-Arevalo, Choi, Stefanski, Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients, Sci Immunol
Ripoll, Gorman, Juskewitch, Vaccine-boosted convalescent plasma therapy for patients with immunosuppression and COVID-19, Blood Adv
Ripoll, Tulledge-Scheitel, Stephenson, Outpatient treatment with concomitant vaccine-boosted convalescent plasma for patients with immunosuppression and COVID-19, mBio
Salazar, Perez, Ashraf, Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma, Am J Pathol
Sattler, Thumfart, Toth, SARS-CoV2 mRNA vaccinespecific B-, T-and humoral responses in adolescents after kidney transplantation, Transpl Int
Schrezenmeier, Rincon-Arevalo, Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccinationspecific humoral and cellular immunity in kidney transplant recipients, JCI Insight
Seidel, Hoffmann, Jahrsdorfer, SARS-CoV-2 vaccination of convalescents boosts neutralization capacity against Omicron subvariants BA.1, BA.2 and BA.5 and can be predicted by anti-S antibody concentrations in serological assays, Front Immunol
Senefeld, Franchini, Mengoli, COVID-19 convalescent plasma for the treatment of immunocompromised patients: a systematic review and meta-analysis, JAMA Netw Open
Sullivan, Gebo, Shoham, Early outpatient treatment for Covid-19 with convalescent plasma, N Engl J Med
Thompson, Henderson, Shah, Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19, JAMA Oncol
Wang, Muecksch, Schaefer-Babajew, Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection, Nature
Yang, Sun, He, Clinical feature of omicron infection in children with inborn errors of immunity in China, Front Immunol
Zohar, Alter, Dissecting antibody-mediated protection against SARS-CoV-2, Nat Rev Immunol
DOI record:
{
"DOI": "10.1016/j.ebiom.2025.105613",
"ISSN": [
"2352-3964"
],
"URL": "http://dx.doi.org/10.1016/j.ebiom.2025.105613",
"alternative-id": [
"S235239642500057X"
],
"article-number": "105613",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eBioMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ebiom.2025.105613"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Hoffmann",
"given": "Simone",
"sequence": "first"
},
{
"affiliation": [],
"family": "Schrezenmeier",
"given": "Eva",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3801-5297",
"affiliation": [],
"authenticated-orcid": false,
"family": "Desmarets",
"given": "Maxime",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halleck",
"given": "Fabian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Durrbach",
"given": "Antoine",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4882-6816",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peters",
"given": "Lynn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremmel",
"given": "Anna-Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seidel",
"given": "Alina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Führer",
"given": "Marita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bachmann",
"given": "Friederike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schrezenmeier",
"given": "Jens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greiner",
"given": "Jochen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Körper",
"given": "Sixten",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hofmann",
"given": "Henrike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ludwig",
"given": "Carolin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vieweg",
"given": "Christiane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jahrsdörfer",
"given": "Bernd",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Budde",
"given": "Klemens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schmidt",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Münch",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joher",
"given": "Nizar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daguindau",
"given": "Etienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grüner",
"given": "Beate",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brunotte",
"given": "Gaëlle",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5106-7369",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vauchy",
"given": "Charline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seifried",
"given": "Erhard",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7186-2482",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bradshaw",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estcourt",
"given": "Lise J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roberts",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Toussirot",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rijnders",
"given": "Bart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tiberghien",
"given": "Pierre",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1222-6659",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schrezenmeier",
"given": "Hubert",
"sequence": "additional"
}
],
"container-title": "eBioMedicine",
"container-title-short": "eBioMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
27
]
],
"date-time": "2025-02-27T19:01:37Z",
"timestamp": 1740682897000
},
"deposited": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T08:22:17Z",
"timestamp": 1741767737000
},
"funder": [
{
"DOI": "10.13039/501100001826",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001826",
"id-type": "DOI"
}
],
"name": "Netherlands Organisation for Health Research and Development"
},
{
"DOI": "10.13039/501100002347",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100002347",
"id-type": "DOI"
}
],
"name": "Federal Ministry of Education and Research Bonn Office"
},
{
"DOI": "10.13039/100010661",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100010661",
"id-type": "DOI"
}
],
"name": "Horizon 2020 Framework Programme"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
19
]
],
"date-time": "2025-03-19T11:03:06Z",
"timestamp": 1742382186004,
"version": "3.38.0"
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2025,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
1
]
],
"date-time": "2025-03-01T00:00:00Z",
"timestamp": 1740787200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
1
]
],
"date-time": "2025-03-01T00:00:00Z",
"timestamp": 1740787200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
8
]
],
"date-time": "2025-02-08T00:00:00Z",
"timestamp": 1738972800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S235239642500057X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S235239642500057X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105613",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
3
]
]
},
"published-print": {
"date-parts": [
[
2025,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Convalescent plasma for people with COVID-19: a living systematic review",
"author": "Iannizzi",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/j.ebiom.2025.105613_bib1",
"volume": "5",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe Covid-19 in older adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ebiom.2025.105613_bib2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2119657",
"article-title": "Early outpatient treatment for Covid-19 with convalescent plasma",
"author": "Sullivan",
"doi-asserted-by": "crossref",
"first-page": "1700",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ebiom.2025.105613_bib3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00545-2",
"article-title": "High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial",
"author": "Alemany",
"doi-asserted-by": "crossref",
"first-page": "278",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.ebiom.2025.105613_bib4",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-29911-3",
"article-title": "Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients",
"author": "Millat-Martinez",
"doi-asserted-by": "crossref",
"first-page": "2583",
"journal-title": "Nat Commun",
"key": "10.1016/j.ebiom.2025.105613_bib5",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.08.005",
"article-title": "Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"first-page": "208",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.ebiom.2025.105613_bib6",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early convalescent plasma for high-risk outpatients with Covid-19",
"author": "Korley",
"doi-asserted-by": "crossref",
"first-page": "1951",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ebiom.2025.105613_bib7",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jamaoncol.2021.1799",
"article-title": "Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19",
"author": "Thompson",
"doi-asserted-by": "crossref",
"first-page": "1167",
"journal-title": "JAMA Oncol",
"key": "10.1016/j.ebiom.2025.105613_bib8",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1038/s41375-022-01511-6",
"article-title": "Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis",
"author": "Hueso",
"doi-asserted-by": "crossref",
"first-page": "1025",
"journal-title": "Leukemia",
"key": "10.1016/j.ebiom.2025.105613_bib9",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3390/v13030436",
"article-title": "Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID-19",
"author": "Biernat",
"doi-asserted-by": "crossref",
"first-page": "436",
"journal-title": "Viruses",
"key": "10.1016/j.ebiom.2025.105613_bib10",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1128/mbio.00400-24",
"article-title": "Outpatient treatment with concomitant vaccine-boosted convalescent plasma for patients with immunosuppression and COVID-19",
"author": "Ripoll",
"doi-asserted-by": "crossref",
"journal-title": "mBio",
"key": "10.1016/j.ebiom.2025.105613_bib11",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1182/bloodadvances.2022008932",
"article-title": "Vaccine-boosted convalescent plasma therapy for patients with immunosuppression and COVID-19",
"author": "Ripoll",
"doi-asserted-by": "crossref",
"first-page": "5951",
"journal-title": "Blood Adv",
"key": "10.1016/j.ebiom.2025.105613_bib12",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1172/JCI155114",
"article-title": "A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia",
"author": "Bar",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.ebiom.2025.105613_bib13",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.18178",
"article-title": "Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial",
"author": "Estcourt",
"doi-asserted-by": "crossref",
"first-page": "1690",
"journal-title": "JAMA",
"key": "10.1016/j.ebiom.2025.105613_bib14",
"volume": "326",
"year": "2021"
},
{
"article-title": "Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial",
"author": "Denkinger",
"first-page": "96",
"journal-title": "Nat Cancer",
"key": "10.1016/j.ebiom.2025.105613_bib15",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1136/bmjmed-2022-000427",
"article-title": "Use of covid-19 convalescent plasma to treat patients admitted to hospital for covid-19 with or without underlying immunodeficiency: open label, randomised clinical trial",
"author": "Lacombe",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Med",
"key": "10.1016/j.ebiom.2025.105613_bib16",
"volume": "2",
"year": "2023"
},
{
"DOI": "10.1136/bmjopen-2022-071277",
"article-title": "Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19 (COVIC-19): protocol for a randomised, open-label trial",
"author": "Desmarets",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/j.ebiom.2025.105613_bib17",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.immuni.2022.09.006",
"article-title": "SARS-CoV-2 in immunocompromised individuals",
"author": "DeWolf",
"doi-asserted-by": "crossref",
"first-page": "1779",
"journal-title": "Immunity",
"key": "10.1016/j.ebiom.2025.105613_bib18",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1126/sciimmunol.abj1031",
"article-title": "Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients",
"author": "Rincon-Arevalo",
"doi-asserted-by": "crossref",
"journal-title": "Sci Immunol",
"key": "10.1016/j.ebiom.2025.105613_bib19",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03696-9",
"article-title": "Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "426",
"journal-title": "Nature",
"key": "10.1016/j.ebiom.2025.105613_bib20",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2023.1170759",
"article-title": "SARS-CoV-2 vaccination of convalescents boosts neutralization capacity against Omicron subvariants BA.1, BA.2 and BA.5 and can be predicted by anti-S antibody concentrations in serological assays",
"author": "Seidel",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/j.ebiom.2025.105613_bib21",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1002/(SICI)1097-0258(19980430)17:8<873::AID-SIM779>3.0.CO;2-I",
"article-title": "Interval estimation for the difference between independent proportions: comparison of eleven methods",
"author": "Newcombe",
"doi-asserted-by": "crossref",
"first-page": "873",
"journal-title": "Stat Med",
"key": "10.1016/j.ebiom.2025.105613_bib22",
"volume": "17",
"year": "1998"
},
{
"DOI": "10.1001/jamanetworkopen.2022.50647",
"article-title": "COVID-19 convalescent plasma for the treatment of immunocompromised patients: a systematic review and meta-analysis",
"author": "Senefeld",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ebiom.2025.105613_bib23",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1038/s41579-023-00878-2",
"article-title": "The evolution of SARS-CoV-2",
"author": "Markov",
"doi-asserted-by": "crossref",
"first-page": "361",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/j.ebiom.2025.105613_bib24",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.3389/fonc.2024.1389345",
"article-title": "Omicron SARS-CoV-2 infection management and outcomes in patients with hematologic disease and recipients of cell therapy",
"author": "Pinana",
"doi-asserted-by": "crossref",
"journal-title": "Front Oncol",
"key": "10.1016/j.ebiom.2025.105613_bib25",
"volume": "14",
"year": "2024"
},
{
"article-title": "Clinical feature of omicron infection in children with inborn errors of immunity in China",
"author": "Yang",
"journal-title": "Front Immunol",
"key": "10.1016/j.ebiom.2025.105613_bib26",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.20411/pai.v10i1.752",
"article-title": "Escape of SARS-CoV-2 variants KP.1.1, LB.1, and KP3.3 from approved monoclonal antibodies",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Pathog Immun",
"key": "10.1016/j.ebiom.2025.105613_bib27",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.3389/fimmu.2022.920210",
"article-title": "BNT162b2 booster vaccination elicits cross-reactive immunity against SARS-CoV-2 variants B.1.1.529 and B.1.617.2 in convalescents of all ages",
"author": "Jahrsdorfer",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/j.ebiom.2025.105613_bib28",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/ti.2022.10677",
"article-title": "SARS-CoV2 mRNA vaccine-specific B-, T- and humoral responses in adolescents after kidney transplantation",
"author": "Sattler",
"doi-asserted-by": "crossref",
"journal-title": "Transpl Int",
"key": "10.1016/j.ebiom.2025.105613_bib29",
"volume": "35",
"year": "2022"
},
{
"DOI": "10.1172/jci.insight.157836",
"article-title": "Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients",
"author": "Schrezenmeier",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight",
"key": "10.1016/j.ebiom.2025.105613_bib30",
"volume": "7",
"year": "2022"
},
{
"article-title": "Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients",
"author": "Osmanodja",
"first-page": "2565",
"journal-title": "J Clin Med",
"key": "10.1016/j.ebiom.2025.105613_bib31",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.isci.2022.104448",
"article-title": "Modeling explains prolonged SARS-CoV-2 nasal shedding relative to lung shedding in remdesivir-treated rhesus macaques",
"author": "Goyal",
"doi-asserted-by": "crossref",
"journal-title": "iScience",
"key": "10.1016/j.ebiom.2025.105613_bib33",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03291-y",
"article-title": "SARS-CoV-2 evolution during treatment of chronic infection",
"author": "Kemp",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Nature",
"key": "10.1016/j.ebiom.2025.105613_bib34",
"volume": "592",
"year": "2021"
},
{
"DOI": "10.1126/science.abf6950",
"article-title": "Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape",
"author": "McCarthy",
"doi-asserted-by": "crossref",
"first-page": "1139",
"journal-title": "Science",
"key": "10.1016/j.ebiom.2025.105613_bib35",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03471-w",
"article-title": "Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma",
"author": "Cele",
"doi-asserted-by": "crossref",
"first-page": "142",
"journal-title": "Nature",
"key": "10.1016/j.ebiom.2025.105613_bib36",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.7212",
"article-title": "Researchers tie severe immunosuppression to chronic COVID-19 and virus variants",
"author": "Abbasi",
"doi-asserted-by": "crossref",
"first-page": "2033",
"journal-title": "JAMA",
"key": "10.1016/j.ebiom.2025.105613_bib37",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"article-title": "Convalescent plasma antibody levels and the risk of death from Covid-19",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "1015",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ebiom.2025.105613_bib38",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1172/JCI152264",
"article-title": "Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19",
"author": "Körper",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.ebiom.2025.105613_bib39",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1172/JCI163657",
"article-title": "One-year follow-up of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients",
"author": "Korper",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.ebiom.2025.105613_bib40",
"volume": "132",
"year": "2022"
},
{
"DOI": "10.1016/j.ajpath.2020.05.014",
"article-title": "Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma",
"author": "Salazar",
"doi-asserted-by": "crossref",
"first-page": "1680",
"journal-title": "Am J Pathol",
"key": "10.1016/j.ebiom.2025.105613_bib41",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.ebiom.2025.105613_bib42",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0359-5",
"article-title": "Dissecting antibody-mediated protection against SARS-CoV-2",
"author": "Zohar",
"doi-asserted-by": "crossref",
"first-page": "392",
"journal-title": "Nat Rev Immunol",
"key": "10.1016/j.ebiom.2025.105613_bib43",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3390/v13081594",
"article-title": "COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors",
"author": "Focosi",
"doi-asserted-by": "crossref",
"first-page": "1594",
"journal-title": "Viruses",
"key": "10.1016/j.ebiom.2025.105613_bib44",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1111/trf.17701",
"article-title": "Safety of COVID-19 convalescent plasma: a definitive systematic review and meta-analysis of randomized controlled trials",
"author": "Franchini",
"doi-asserted-by": "crossref",
"first-page": "388",
"journal-title": "Transfusion",
"key": "10.1016/j.ebiom.2025.105613_bib45",
"volume": "64",
"year": "2024"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S235239642500057X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "113"
}