Real-world evidence of sotrovimab effectiveness for preventing severe outcomes in patients with COVID-19: A quality improvement propensity matched retrospective cohort study of a pan-provincial program in Alberta, Canada
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2024.107136, Jun 2024
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
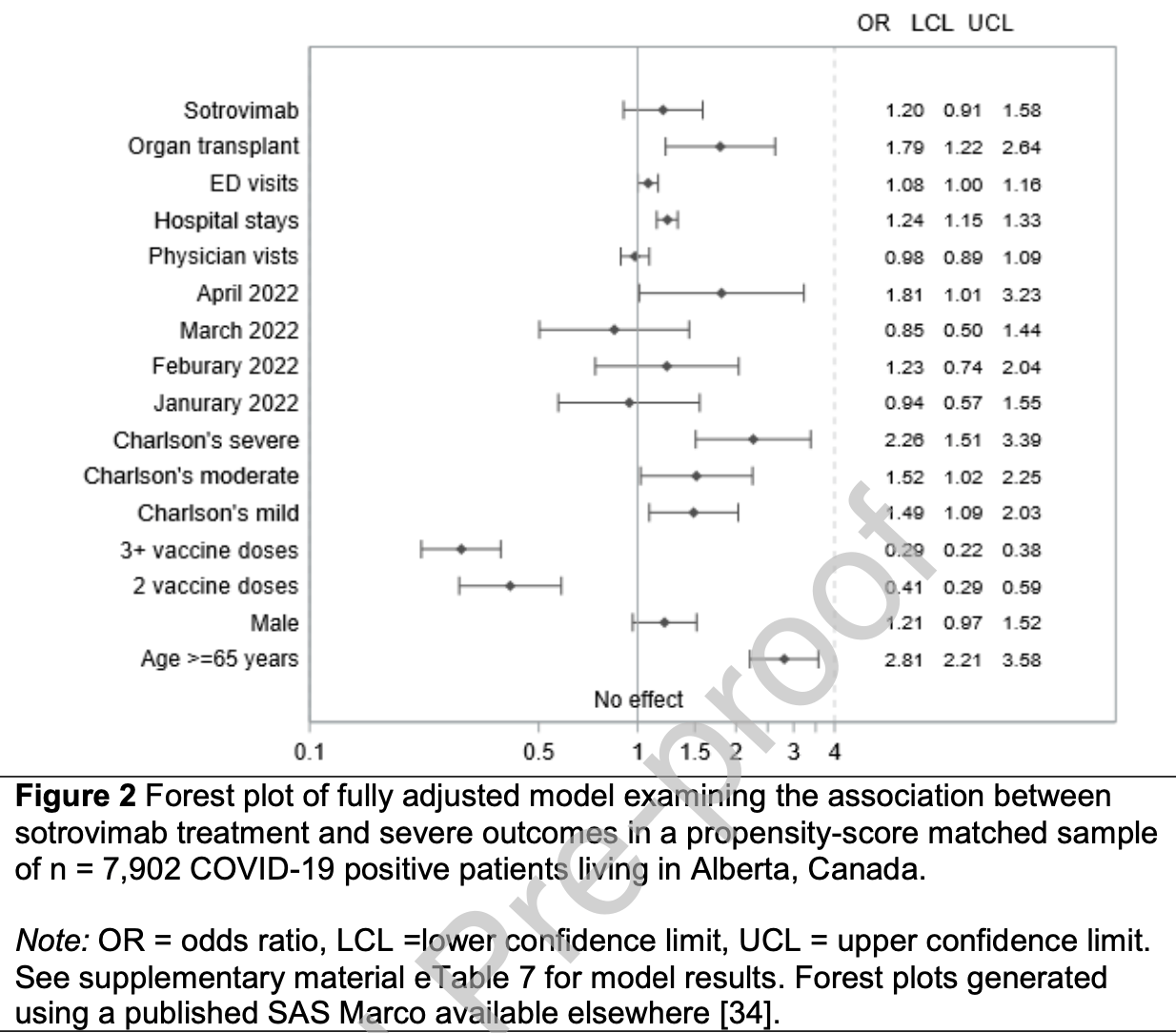
Retrospective propensity-matched study of 22,289 high-risk COVID-19 outpatients in Canada, showing no significant difference in combined hospitalization/mortality with sotrovimab treatment. In a subgroup analysis of patients with no comorbidities, sotrovimab was associated with lower odds of severe outcomes. The study period included Omicron BA.1 and BA.2 variants, which may have contributed to the reduced efficacy compared to earlier studies.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
|
risk of death/hospitalization, 20.0% higher, OR 1.20, p = 0.20, treatment 1,603, control 6,299, adjusted per study, propensity score matching, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Farmer et al., 19 Jun 2024, retrospective, Canada, peer-reviewed, 14 authors, study period 15 December, 2021 - 30 April, 2022.
Contact: gregory.farmer@albertahealthservices.ca.
Abstract: Journal Pre-proof
Real-world evidence of sotrovimab effectiveness for preventing
severe outcomes in patients with COVID-19: A quality improvement
propensity matched retrospective cohort study of a pan-provincial
program in Alberta, Canada
Gregory Farmer , Khokan C. Sikdar , TKT Lo , John Conly ,
Jeremy Slobodan , Jordan Ross , Samantha James ,
Hussain Usman , Kyle Kemp , Kristi Baker , Karen Doucette ,
Cheri Nijssen-Jordan , Lynora M. Saxinger , A. Mark Joffe
PII:
DOI:
Reference:
S1201-9712(24)00207-8
https://doi.org/10.1016/j.ijid.2024.107136
IJID 107136
To appear in:
International Journal of Infectious Diseases
Received date:
Revised date:
Accepted date:
11 January 2024
21 May 2024
11 June 2024
Please cite this article as: Gregory Farmer , Khokan C. Sikdar , TKT Lo , John Conly ,
Jeremy Slobodan , Jordan Ross , Samantha James , Hussain Usman , Kyle Kemp , Kristi Baker ,
Karen Doucette , Cheri Nijssen-Jordan , Lynora M. Saxinger , A. Mark Joffe , Real-world evidence of sotrovimab effectiveness for preventing severe outcomes in patients with COVID19: A quality improvement propensity matched retrospective cohort study of a pan-provincial
program in Alberta, Canada, International Journal of Infectious Diseases (2024), doi:
https://doi.org/10.1016/j.ijid.2024.107136
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition
of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of
record. This version will undergo additional copyediting, typesetting and review before it is published
in its final form, but we are providing this version to give early visibility of the article. Please note that,
during the production process, errors may be discovered which could affect the content, and all legal
disclaimers that apply to the journal pertain.
© 2024 Published by Elsevier Ltd on behalf of International Society for Infectious Diseases.
This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/)
Highlights:
Post-marketing surveillance of sotrovimab in Canada is limited.
Sotrovimab was not associated with improved outcomes.
Study results were consistent across different analytic methodologies.
Study findings need to be confirmed in other Canadian provinces.
Sotrovimab effectiveness
Title: Real-world evidence of sotrovimab effectiveness for preventing severe outcomes in
patients with COVID-19: A quality improvement propensity matched retrospective cohort study
of a pan-provincial program in Alberta, Canada
Authors: Gregory Farmer, MSc1; Khokan C. Sikdar, PhD1,5; TKT Lo, PhD1; John Conly, MD,
DSc2,8; Jeremy Slobodan, BSP3; Jordan Ross, MPH1; Samantha James, MPH1; Hussain Usman,
M.B.B.S, MSc, Dr.PH1; Kyle Kemp, PhD1; Kristi Baker, PhD4; Karen Doucette, MD, MSc6,8;
Cheri Nijssen-Jordan, MD, MBA7; Lynora, M. Saxinger, MD6,8; A. Mark Joffe, MD6,8.
Author affiliations: 1Department of Provincial Population and Public Health (PPPH), Alberta
Health Services, Edmonton, Canada; 2 Department of Medicine, Division of Infectious Diseases,
Cumming School of Medicine, University of Calgary, Calgary, Canada; 3Department of Drug
Utilization, Information and Stewardship, Alberta Health Services, Edmonton, Canada;
4
Department of Oncology, Division of Experimental Oncology, University of Alberta, Edmonton,
Canada; 5Department of Community Health Sciences, Cumming School of Medicine, University
of..
DOI record:
{
"DOI": "10.1016/j.ijid.2024.107136",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2024.107136",
"alternative-id": [
"S1201971224002078"
],
"article-number": "107136",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world evidence of sotrovimab effectiveness for preventing severe outcomes in patients with COVID-19: A quality improvement propensity matched retrospective cohort study of a pan-provincial program in Alberta, Canada"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2024.107136"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Farmer",
"given": "Gregory",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sikdar",
"given": "Khokan C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lo",
"given": "TKT",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conly",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slobodan",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ross",
"given": "Jordan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "James",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Usman",
"given": "Hussain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kemp",
"given": "Kyle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baker",
"given": "Kristi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doucette",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nijssen-Jordan",
"given": "Cheri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saxinger",
"given": "Lynora M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joffe",
"given": "A. Mark",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
6,
14
]
],
"date-time": "2024-06-14T21:02:25Z",
"timestamp": 1718398945000
},
"deposited": {
"date-parts": [
[
2024,
6,
14
]
],
"date-time": "2024-06-14T21:02:25Z",
"timestamp": 1718398945000
},
"indexed": {
"date-parts": [
[
2024,
6,
15
]
],
"date-time": "2024-06-15T00:24:43Z",
"timestamp": 1718411083842
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:00:00Z",
"timestamp": 1717200000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:00:00Z",
"timestamp": 1717200000000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2024,
6,
12
]
],
"date-time": "2024-06-12T00:00:00Z",
"timestamp": 1718150400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971224002078?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971224002078?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "107136",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
6
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971224002078"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-world evidence of sotrovimab effectiveness for preventing severe outcomes in patients with COVID-19: A quality improvement propensity matched retrospective cohort study of a pan-provincial program in Alberta, Canada",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
