Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-1
et al., Journal of Medical Virology, doi:10.1002/jmv.26880, Feb 2021
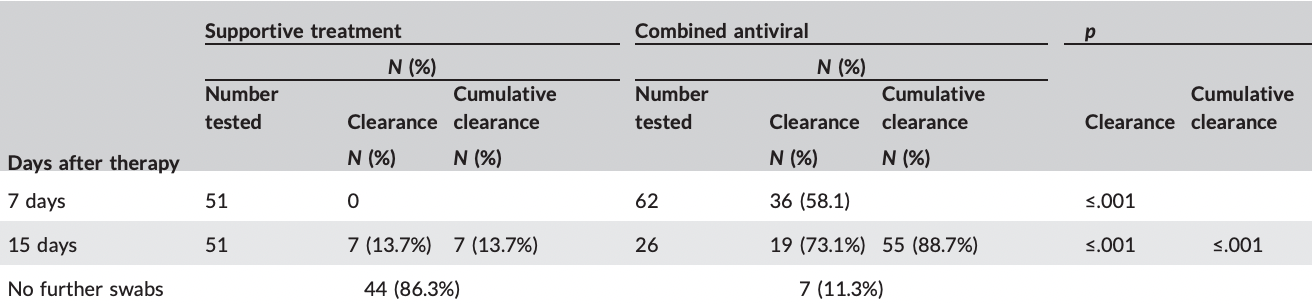
Non-randomized controlled trial with 62 mild and early moderate patients with home treatment with ivermectin + nitazoxanide + ribavirin + zinc, showing significantly faster viral clearance.
Study covers nitazoxanide and ivermectin.
|
risk of no viral clearance, 86.9% lower, RR 0.13, p < 0.001, treatment 7 of 62 (11.3%), control 44 of 51 (86.3%), NNT 1.3, day 15.
|
|
risk of no viral clearance, 58.1% lower, RR 0.42, p < 0.001, treatment 26 of 62 (41.9%), control 51 of 51 (100.0%), NNT 1.7, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Elalfy et al., 16 Feb 2021, retrospective, Egypt, peer-reviewed, 15 authors, this trial uses multiple treatments in the treatment arm (combined with ivermectin, ribavirin, and zinc) - results of individual treatments may vary.
Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID‐19
Journal of Medical Virology, doi:10.1002/jmv.26880
This trial compared the rate and time of viral clearance in subjects receiving a combination of nitazoxanide, ribavirin, and ivermectin plus Zinc versus those receiving supportive treatment. This non-randomized controlled trial included 62 patients on the triple combination treatment versus 51 age-and sex-matched patients on routine supportive treatment. all of them confirmed cases by positive reverse-transcription polymerase chain reaction of a nasopharyngeal swab. Trial results showed that the clearance rates were 0% and 58.1% on the 7th day and 13.7% and 73.1% on the 15th day in the supportive treatment and combined antiviral groups, respectively. The cumulative clearance rates on the 15th day are 13.7% and 88.7% in the supportive treatment and combined antiviral groups, respectively. This trial concluded by stating that the combined use of nitazoxanide, ribavirin, and ivermectin plus zinc supplement effectively cleared the SARS-COV2 from the nasopharynx in a shorter time than symptomatic therapy.
lower than effective in vitro concentrations against SARS-CoV-2. 43 A recent phase III clinical trial in dengue patients (DNV) in Thailand revealed that lower doses of ivermectin can be effective. A oncedaily dose of 400 µg/kg for 3 days was found to be safe but did not produce any clinical benefit, and showed a modest and indirect in vivo effect against DNV. 44 The antioxidant, anti-inflammatory, immunomodulatory and antiviral activities of Zn are well known. Its antiviral effect is mediated by suppressing RNA-dependent RNA polymerase (RdRp). 45 Zinc ions (Zn 2+ ) are closely involved in the normal development, differentiation, and function of immune cells, thus considered critical for generating both innate and acquired (humoral) antiviral responses. 32 The synergistic effect of zinc, if combined with antiviral treatment, has been proved previously with hepatitis C virus, human papillomavirus, viral diarrhea in children, and human immunodeficiency virus. 46-49 Short-term treatment with zinc in therapeutic doses is completely safe. Zn toxicity rarely occurs in very sporadic cases unlike many other metal ions with similar chemical properties. 50 Study limitation: the groups were not randomized and the drug combination does not have an established in vitro mechanism of action and remains exploratory.
| CONCLUSION The results of this study confirm that combined use of nitazoxanide, ribavirin, and ivermectin plus zinc supplement effectively cleared the SARS-COV2 from..
References
Al-Tawfiq, Momattin, Dib, Memish, Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study, Int J Infect Dis
Booth, Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area, JAMA
Caly, Druce, Catton, Jans, Wagstaff, The FDAapproved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Campbell, Benz, Ivermectin: a review of efficacy and safety, J Vet Pharmacol Ther
Cao, Forrest, Zhang, A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs, Antiviral Res
Chong, Song, Seo, Antiviral treatment guidelines for Middle East respiratory syndrome, Infect Chemother
Crotty, Cameron, Andino, RNA virus error catastrophe: direct molecular test by using ribavirin, Proc Natl Acad Sci U S A
Crump, Ōmura, Ivermectin, 'wonder drug' from Japan: the human use perspective, Proc Jpn Acad Ser B
Fox, Saravolatz, Nitazoxanide: a new thiazolide antiparasitic agent, Clin Infect Dis
Graci, Cameron, Mechanisms of action of ribavirin against distinct viruses, Rev Med Virol
Habib, Ali, Zouaoui, Taha, Mohammed et al., Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection, BMC Infect Dis
Hong, Kim, Song, Choi, Lee et al., Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice, Int Immunopharmacol
Hultgren, Milich, Weiland, Sallberg, The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses, J Gen Virol
Hung, Lung, Tso, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet
Khalid, Rabiah, Khan, Mobeireek, Butt et al., Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases, Antivir Ther
Kobayashi, Nakatsuka, Shimizu, Ribavirin modulates the conversion of human CD4(+) CD25(-) T cell to CD4(+) CD25(+) FOXP3(+) T cell via suppressing interleukin-10-producing regulatory T cell, Immunology
Kumar, Kubota, Chernov, Kasuya, Potential role of zinc supplementation in prophylaxis and treatment of COVID-19, Med Hypotheses
Lee, Hui, Wu, A major outbreak of severe acute respiratory syndrome in Hong Kong, N Engl J Med
Li, Ping, Yu, Dynamic changes in CD45RA(-)Foxp3(high) regulatory T-cells in chronic hepatitis C patients during antiviral therapy, Int J Infect Dis
Lundberg, Pinkham, Baer, Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication, Antiviral Res
Lv, Liu, Wang, Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo, Antiviral Res
Overbeck, Rink, Haase, Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases, Arch Immunol Ther Exp
Peiris, Chu, Cheng, Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study, Lancet
Poutanen, Low, Henry, Identification of severe acute respiratory syndrome in Canada, N Engl J Med
Rahman, Idid, Can Zn be a critical element in COVID-19 treatment?, Biol Trace Elem Res
Raza, Shahin, Zhai, Ivermectin inhibits bovine herpesvirus 1 DNA polymerase nuclear import and interferes with viral replication, Microorganisms
Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus, J Infect Public Health
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, JAMA
Tam, Pai, Bard, Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile, J Hepatol
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Lv, Wang, Qiu, Yang, Ivermectin treatment inhibits the replication of porcine circovirus 2 (PCV2) in vitro and mitigates the impact of viral infection in piglets, Virus Res
Yousefi, Valizadeh, Ghaffari, Vahedi, Karbalaei et al., A global treatments for coronaviruses including COVID-19, J Cell Physiol
Zeng, Xu, He, Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferonalpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol, Chin Med J
Zhong, Wang, Zhang, Efficacy and safety of current therapeutic options for COVID-19-lessons to be learnt from SARS
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.1002/jmv.26880",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.26880",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:label /><jats:p>This trial compared the rate and time of viral clearance in subjects receiving a combination of nitazoxanide, ribavirin, and ivermectin plus Zinc versus those receiving supportive treatment. This non‐randomized controlled trial included 62 patients on the triple combination treatment versus 51 age‐ and sex‐matched patients on routine supportive treatment. all of them confirmed cases by positive reverse‐transcription polymerase chain reaction of a nasopharyngeal swab. Trial results showed that the clearance rates were 0% and 58.1% on the 7th day and 13.7% and 73.1% on the 15th day in the supportive treatment and combined antiviral groups, respectively. The cumulative clearance rates on the 15th day are 13.7% and 88.7% in the supportive treatment and combined antiviral groups, respectively. This trial concluded by stating that the combined use of nitazoxanide, ribavirin, and ivermectin plus zinc supplement effectively cleared the SARS‐COV2 from the nasopharynx in a shorter time than symptomatic therapy.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/jmv.26880"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-12-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-02-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-03-11"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5602-0989",
"affiliation": [
{
"name": "Tropical Medicine and Hepatology Department, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"authenticated-orcid": false,
"family": "Elalfy",
"given": "Hatem",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Tropical Medicine, Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Besheer",
"given": "Tarek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Tropical Medicine, Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "El‐Mesery",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Public Health and Preventive Medicine, Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "El‐Gilany",
"given": "Abdel‐Hady",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Tropical Medicine, Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Soliman",
"given": "Mahmoud Abdel‐Aziz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Tropical Medicine, Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Alhawarey",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tropical Medicine and Hepatology Department, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Alegezy",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chest Department Mansoura University Mansoura Egypt"
}
],
"family": "Elhadidy",
"given": "Tamer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chest Medicine Department Mansoura University Mansoura Egypt"
}
],
"family": "Hewidy",
"given": "Asem A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pathology, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Zaghloul",
"given": "Hossam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Neamatallah",
"given": "Mustafa Ahmed Mohamed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pathology, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Raafat",
"given": "Douaa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pathology, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "El‐Emshaty",
"given": "Wafaa M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pathology, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "Abo El Kheir",
"given": "Nermin Y.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tropical Medicine and Hepatology Department, Mansoura Faculty of Medicine Mansoura University Mansoura Egypt"
}
],
"family": "El‐Bendary",
"given": "Mahmoud",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
17
]
],
"date-time": "2021-02-17T09:55:19Z",
"timestamp": 1613555719000
},
"deposited": {
"date-parts": [
[
2023,
8,
29
]
],
"date-time": "2023-08-29T12:07:38Z",
"timestamp": 1693310858000
},
"funder": [
{
"DOI": "10.13039/501100009367",
"award": [
"RP.20.05.69",
"mu‐med‐2020‐26"
],
"doi-asserted-by": "publisher",
"name": "Mansoura University"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T14:36:49Z",
"timestamp": 1712155009814
},
"is-referenced-by-count": 44,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
3,
11
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
11
]
],
"date-time": "2021-03-11T00:00:00Z",
"timestamp": 1615420800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.26880",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jmv.26880",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.26880",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "3176-3183",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
3,
11
]
]
},
"published-online": {
"date-parts": [
[
2021,
3,
11
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_2_1"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_3_1"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review",
"author": "Sanders JM",
"first-page": "1824",
"issue": "18",
"journal-title": "JAMA",
"key": "e_1_2_12_4_1",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1086/428839",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_5_1"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_6_1"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_7_1"
},
{
"DOI": "10.1016/j.intimp.2012.03.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_8_1"
},
{
"DOI": "10.1016/j.antiviral.2014.11.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_9_1"
},
{
"DOI": "10.1073/pnas.111085598",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_10_1"
},
{
"DOI": "10.1002/rmv.483",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_11_1"
},
{
"DOI": "10.1099/0022-1317-79-10-2381",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_12_1"
},
{
"DOI": "10.1111/imm.12005",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_13_1"
},
{
"DOI": "10.1016/j.ijid.2016.02.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_14_1"
},
{
"DOI": "10.1016/S0168-8278(99)80093-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_15_1"
},
{
"DOI": "10.1016/j.ijid.2013.12.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_16_1"
},
{
"DOI": "10.1001/jama.289.21.JOC30885",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_17_1"
},
{
"DOI": "10.3947/ic.2015.47.3.212",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_18_1"
},
{
"DOI": "10.1186/s12879-019-4555-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_19_1"
},
{
"DOI": "10.3851/IMP2792",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_20_1"
},
{
"DOI": "10.1056/NEJMoa030685",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_21_1"
},
{
"DOI": "10.1016/S0140-6736(03)13412-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_22_1"
},
{
"DOI": "10.1056/NEJMoa030634",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_23_1"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_24_1"
},
{
"DOI": "10.1097/CM9.0000000000000790",
"article-title": "Comparative effectiveness and safety of ribavirin plus interferon‐alpha, lopinavir/ritonavir plus interferon‐alpha, and ribavirin plus lopinavir/ritonavir plus interferon‐alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol",
"author": "Zeng YM",
"doi-asserted-by": "crossref",
"first-page": "1132",
"issue": "9",
"journal-title": "Chin Med J",
"key": "e_1_2_12_25_1",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2885.1984.tb00872.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_26_1"
},
{
"DOI": "10.2183/pjab.87.13",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_27_1"
},
{
"DOI": "10.1016/j.antiviral.2018.09.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_28_1"
},
{
"DOI": "10.1016/j.virusres.2019.01.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_29_1"
},
{
"DOI": "10.3390/microorganisms8030409",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_30_1"
},
{
"DOI": "10.1016/j.antiviral.2013.10.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_31_1"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_32_1"
},
{
"DOI": "10.1007/s00005-008-0003-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_33_1"
},
{
"DOI": "10.1016/j.mehy.2020.109848",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_34_1"
},
{
"article-title": "Can Zn be a critical element in COVID‐19 treatment?",
"author": "Rahman MT",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "e_1_2_12_35_1",
"volume": "199",
"year": "2020"
},
{
"key": "e_1_2_12_36_1",
"unstructured": "Clinical management of COVID‐19 interim guidance. May 2020.https://www.who.int/publications/i/item/clinical-management-of-covid-19"
},
{
"DOI": "10.1002/jcp.29785",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_37_1"
},
{
"DOI": "10.1016/j.phrs.2020.104872",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_38_1"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_39_1"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_40_1"
},
{
"DOI": "10.1016/j.phrs.2020.104874",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_41_1"
},
{
"DOI": "10.1186/s43141-020-00055-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_42_1"
},
{
"DOI": "10.1186/s12936-017-1801-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_43_1"
},
{
"DOI": "10.1177/009127002237994",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_44_1"
},
{
"article-title": "Efficacy and Safety of Ivermectin against dengue Infection: a phase III, randomized, double‐blind, placebo‐controlled trial",
"author": "Yamasmith E.",
"journal-title": "The 34th Annual Meeting of the Royal College of Physicians of Thailand‐ ‘Internal Medicine and One Health’: Chonburi, Thailand. Registry",
"key": "e_1_2_12_45_1",
"year": "2018"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_46_1"
},
{
"DOI": "10.1016/j.bbrc.2016.12.093",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_47_1"
},
{
"DOI": "10.3109/09546634.2010.500324",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_48_1"
},
{
"DOI": "10.1093/ajcn/72.6.1516",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_49_1"
},
{
"DOI": "10.7883/yoken.66.469",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_50_1"
},
{
"article-title": "Zinc and COVID‐19: basis of current clinical trials",
"author": "Pal A",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "e_1_2_12_51_1",
"year": "2020"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.26880"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID‐19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "93"
}