Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study
et al., Pulmonary Pharmacology & Therapeutics, doi:10.1016/j.pupt.2023.102212, RBR-8r9hy8f, Jun 2023
59th treatment shown to reduce risk in
October 2025, now with p = 0.0077 from 3 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
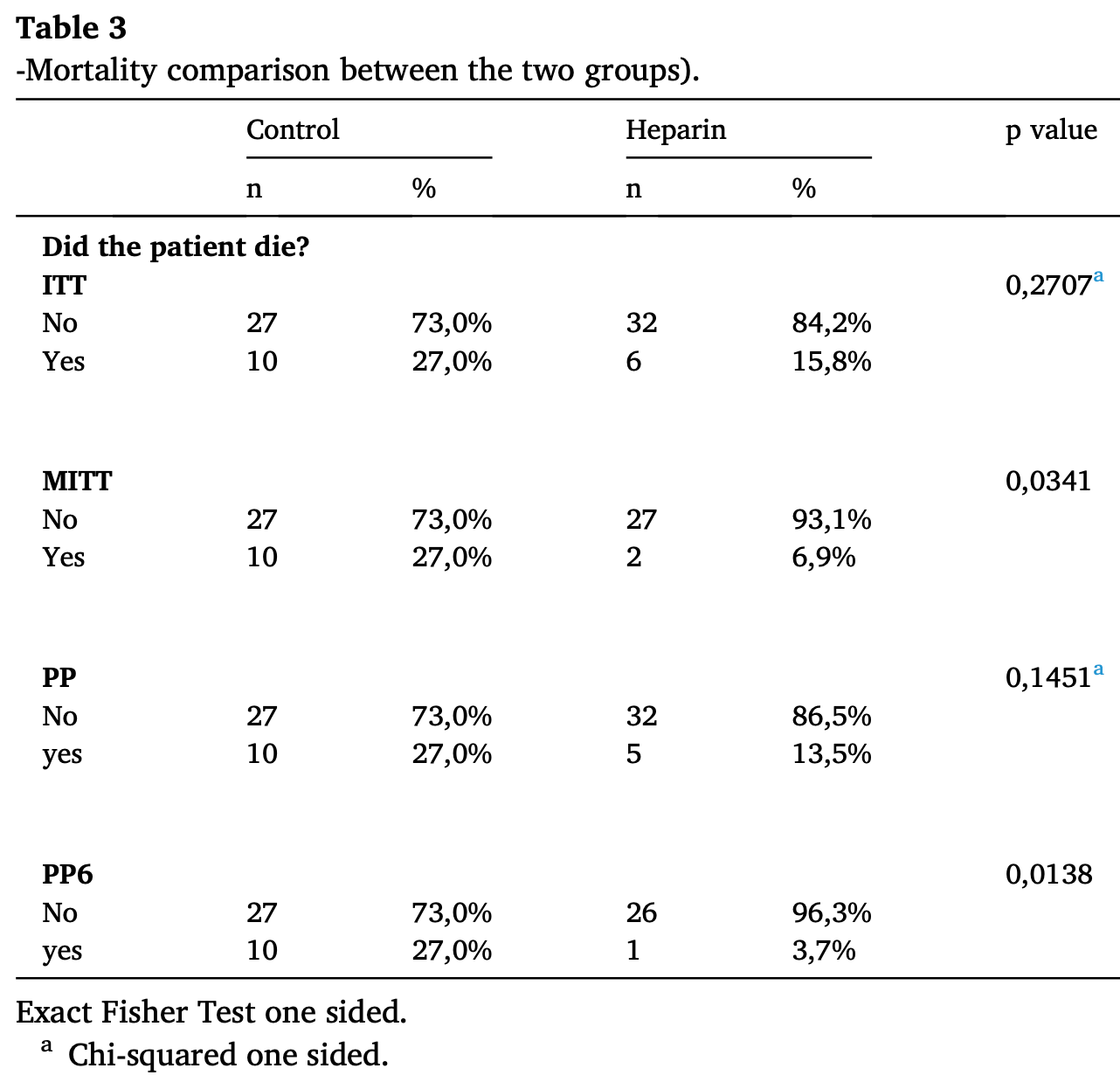
RCT 75 hospitalized patients with COVID-19 showing reduced mortality with inhaled nebulized unfractionated heparin (UFH).
Although the 42% lower mortality is not statistically significant, it is consistent with the significant 50% lower mortality [17‑70%] from meta-analysis of the 3 mortality results to date.
|
risk of death, 41.6% lower, RR 0.58, p = 0.27, treatment 6 of 38 (15.8%), control 10 of 37 (27.0%), NNT 8.9.
|
|
risk of mechanical ventilation, 41.6% lower, RR 0.58, p = 0.27, treatment 6 of 38 (15.8%), control 10 of 37 (27.0%), NNT 8.9.
|
|
hospitalization time, 4.8% lower, relative time 0.95, p = 0.80, treatment mean 12.0 (±9.7) n=38, control mean 12.6 (±11.2) n=38.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
DeNucci et al., 30 Jun 2023, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, 12 authors, study period 25 February, 2021 - 14 July, 2021, trial RBR-8r9hy8f.
Contact: carsver@atcgen.com.br.
Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study
Pulmonary Pharmacology & Therapeutics, doi:10.1016/j.pupt.2023.102212
There is a strong scientific rationale to use nebulised unfractionated heparin (UFH) in treating patients with COVID-19. This pilot study investigated whether nebulised UFH was safe and had any impact on mortality, length of hospitalisation and clinical progression, in the treatment of hospitalised patients with COVID-19. This parallel group, open label, randomised trial included adult patients with confirmed SARS-CoV-2 infection admitted to two hospitals in Brazil. One hundred patients were planned to be randomised to either "standard of care" (SOC) or SOC plus nebulized UFH. The trial was stopped after randomisation of 75 patients due to falling COVID-19 hospitalisation rates. Significance tests were 1-sided test (10% significance level). The key analysis populations were intention to treat (ITT) and modified ITT (mITT) which excluded (from both arms) subjects admitted to ITU or who died within 24 h of randomisation. In the ITT population (n = 75), mortality was numerically lower for nebulised UFH (6 out of 38 patients; 15.8%) versus SOC (10 out of 37 patients; 27.0%), but not statistically significant; odds ratio (OR) 0.51, p = 0.24. However, in the mITT population, nebulised UFH reduced mortality (OR 0.2, p = 0.035). Length of hospital stay was similar between groups, but at day 29, there was a greater improvement in ordinal score following treatment with UFH in the ITT and mITT populations (p = 0.076 and p = 0.012 respectively), while mechanical ventilation rates were lower with UFH in the mITT population (OR 0.31; p = 0.08). Nebulised UFH did not cause any significant adverse events. In conclusion, nebulised UFH added to SOC in hospitalised patients with COVID-19 was well tolerated and showed clinical benefit, particularly in patients who received at least 6 doses of heparin.
Author statement We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. The corresponding author is responsible for ensuring that the descriptions are accurate and agreed by all authors. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual
References
Ciceri, Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis, Crit Care Resusc
Clausen, SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2, Cell
Dahlberg, Clinical versus statistical significance in studies of thoracic malignancies, J. Thorac. Oncol
Dixon, A trial of nebulised heparin to limit lung injury following cardiac surgery, Anaesth. Intensive Care
Dixon, Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial, Lancet Respir. Med
Dixon, Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial, Crit. Care
Dixon, Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury, Crit. Care
Mulloy, Pharmacology of heparin and related drugs, Pharmacol. Rev
Shute, Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: a pilot study, Pulm. Pharmacol. Ther
Takayama, Endo, Otomo, Anticoagulation therapy using unfractionated heparin at a therapeutic dose for coronavirus disease 2019 patients with severe pneumonia: a retrospective historical control study, Acute Med Surg
Tandon, Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives, J. Virol
Thachil, Lessons Learnt from COVID-19 Coagulopathy
Tree, Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations, Br. J. Pharmacol
Van Haren, INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies, Br. J. Clin. Pharmacol
Van Haren, Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: a multicentre case series of 98 patients, Br. J. Clin. Pharmacol
Van Haren, Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence, Crit. Care
DOI record:
{
"DOI": "10.1016/j.pupt.2023.102212",
"ISSN": [
"1094-5539"
],
"URL": "http://dx.doi.org/10.1016/j.pupt.2023.102212",
"alternative-id": [
"S109455392300024X"
],
"article-number": "102212",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Pulmonary Pharmacology & Therapeutics"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.pupt.2023.102212"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "DeNucci",
"given": "Gilberto",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wilkinson",
"given": "Tom",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8676-5431",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sverdloff",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babadopulos",
"given": "Tainah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woodcock",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shute",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Renato Guazelli",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gerbase",
"given": "Luis Frederico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourão",
"given": "Paulo A.S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Dave",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Haren",
"given": "Frank M.P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Page",
"given": "Clive",
"sequence": "additional"
}
],
"container-title": "Pulmonary Pharmacology & Therapeutics",
"container-title-short": "Pulmonary Pharmacology & Therapeutics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.com",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.jp",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
27
]
],
"date-time": "2023-03-27T11:51:25Z",
"timestamp": 1679917885000
},
"deposited": {
"date-parts": [
[
2025,
10,
21
]
],
"date-time": "2025-10-21T21:31:24Z",
"timestamp": 1761082284000
},
"funder": [
{
"DOI": "10.13039/501100023262",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100023262",
"id-type": "DOI"
}
],
"name": "Jon Moulton Charity Trust"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
23
]
],
"date-time": "2025-10-23T21:07:39Z",
"timestamp": 1761253659641,
"version": "build-2065373602"
},
"is-referenced-by-count": 9,
"issued": {
"date-parts": [
[
2023,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S109455392300024X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S109455392300024X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102212",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1186/s13054-020-03148-2",
"article-title": "Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence",
"author": "van Haren",
"doi-asserted-by": "crossref",
"first-page": "454",
"issue": "1",
"journal-title": "Crit. Care",
"key": "10.1016/j.pupt.2023.102212_bib1",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1186/cc9269",
"article-title": "Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury",
"author": "Dixon",
"doi-asserted-by": "crossref",
"first-page": "445",
"issue": "5",
"journal-title": "Crit. Care",
"key": "10.1016/j.pupt.2023.102212_bib2",
"volume": "14",
"year": "2010"
},
{
"DOI": "10.1186/cc9286",
"article-title": "Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial",
"author": "Dixon",
"doi-asserted-by": "crossref",
"first-page": "R180",
"issue": "5",
"journal-title": "Crit. Care",
"key": "10.1016/j.pupt.2023.102212_bib3",
"volume": "14",
"year": "2010"
},
{
"DOI": "10.1177/0310057X1604400106",
"article-title": "A trial of nebulised heparin to limit lung injury following cardiac surgery",
"author": "Dixon",
"doi-asserted-by": "crossref",
"first-page": "28",
"issue": "1",
"journal-title": "Anaesth. Intensive Care",
"key": "10.1016/j.pupt.2023.102212_bib4",
"volume": "44",
"year": "2016"
},
{
"DOI": "10.1016/S2213-2600(20)30470-7",
"article-title": "Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial",
"author": "Dixon",
"doi-asserted-by": "crossref",
"first-page": "360",
"issue": "4",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.pupt.2023.102212_bib5",
"volume": "9",
"year": "2021"
},
{
"article-title": "Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis",
"author": "Ciceri",
"first-page": "95",
"issue": "2",
"journal-title": "Crit Care Resusc",
"key": "10.1016/j.pupt.2023.102212_bib6",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1016/j.pupt.2017.10.001",
"article-title": "Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: a pilot study",
"author": "Shute",
"doi-asserted-by": "crossref",
"first-page": "88",
"journal-title": "Pulm. Pharmacol. Ther.",
"key": "10.1016/j.pupt.2023.102212_bib7",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1016/j.cell.2020.09.033",
"article-title": "SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2",
"author": "Clausen",
"doi-asserted-by": "crossref",
"first-page": "1043",
"issue": "4",
"journal-title": "Cell",
"key": "10.1016/j.pupt.2023.102212_bib8",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1111/bph.15304",
"article-title": "Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations",
"author": "Tree",
"doi-asserted-by": "crossref",
"first-page": "626",
"issue": "3",
"journal-title": "Br. J. Pharmacol.",
"key": "10.1016/j.pupt.2023.102212_bib9",
"volume": "178",
"year": "2021"
},
{
"DOI": "10.1124/pr.115.011247",
"article-title": "Pharmacology of heparin and related drugs",
"author": "Mulloy",
"doi-asserted-by": "crossref",
"first-page": "76",
"issue": "1",
"journal-title": "Pharmacol. Rev.",
"key": "10.1016/j.pupt.2023.102212_bib10",
"volume": "68",
"year": "2016"
},
{
"DOI": "10.1128/JVI.01987-20",
"article-title": "Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives",
"author": "Tandon",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "J. Virol.",
"key": "10.1016/j.pupt.2023.102212_bib11",
"volume": "95",
"year": "2021"
},
{
"author": "Thachil",
"key": "10.1016/j.pupt.2023.102212_bib12",
"series-title": "Lessons Learnt from COVID-19 Coagulopathy",
"year": "2021"
},
{
"DOI": "10.1111/bcp.14714",
"article-title": "INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies",
"author": "van Haren",
"doi-asserted-by": "crossref",
"first-page": "3075",
"issue": "8",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "10.1016/j.pupt.2023.102212_bib13",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1016/j.jtho.2020.06.007",
"article-title": "Clinical versus statistical significance in studies of thoracic malignancies",
"author": "Dahlberg",
"doi-asserted-by": "crossref",
"first-page": "1406",
"issue": "9",
"journal-title": "J. Thorac. Oncol.",
"key": "10.1016/j.pupt.2023.102212_bib14",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1111/bcp.15212",
"article-title": "Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: a multicentre case series of 98 patients",
"author": "van Haren",
"doi-asserted-by": "crossref",
"first-page": "2802",
"issue": "6",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "10.1016/j.pupt.2023.102212_bib15",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1002/ams2.679",
"article-title": "Anticoagulation therapy using unfractionated heparin at a therapeutic dose for coronavirus disease 2019 patients with severe pneumonia: a retrospective historical control study",
"author": "Takayama",
"doi-asserted-by": "crossref",
"first-page": "e679",
"issue": "1",
"journal-title": "Acute Med Surg",
"key": "10.1016/j.pupt.2023.102212_bib16",
"volume": "8",
"year": "2021"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S109455392300024X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "80"
}
