Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00377-5, COV-AID, NCT04330638, Dec 2021
RCT 342 hospitalized COVID-19 patients with hypoxia and signs of cytokine release syndrome in Belgium, showing no significant difference in time to clinical improvement, mortality, or other outcomes with IL-1 blockade (anakinra) or IL-6 blockade (siltuximab, tocilizumab) compared to standard of care.
|
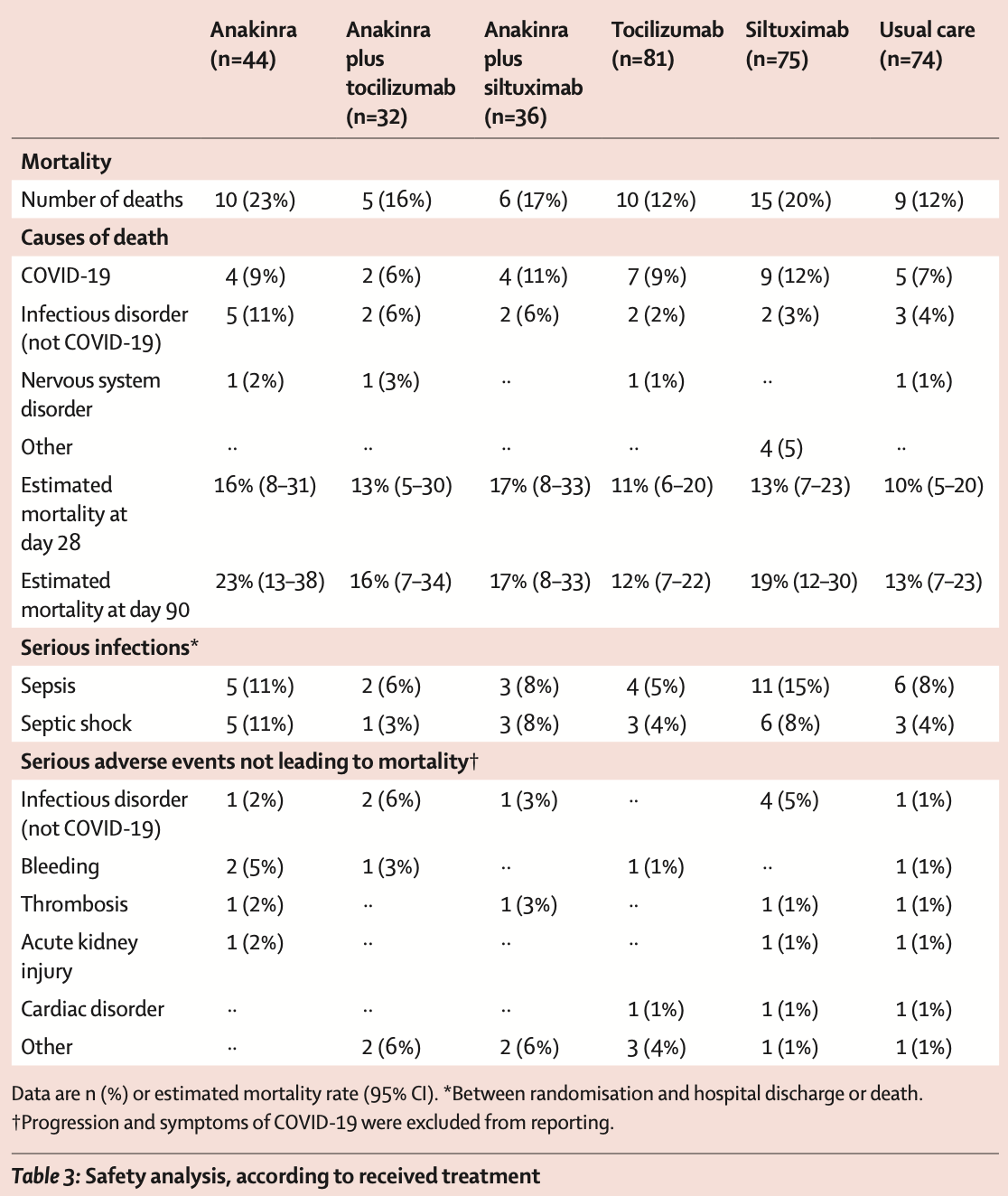
risk of death, 64.4% higher, RR 1.64, p = 0.27, treatment 15 of 75 (20.0%), control 9 of 74 (12.2%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Declercq et al., 31 Dec 2021, Randomized Controlled Trial, Belgium, peer-reviewed, median age 65.0, 43 authors, study period 4 April, 2020 - 6 December, 2020, average treatment delay 10.0 days, trial NCT04330638 (history) (COV-AID).
Contact: bart.lambrecht@ugent.be.
Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00377-5
Background Infections with SARS-CoV-2 continue to cause significant morbidity and mortality. Interleukin (IL)-1 and IL-6 blockade have been proposed as therapeutic strategies in COVID-19, but study outcomes have been conflicting. We sought to study whether blockade of the IL-6 or IL-1 pathway shortened the time to clinical improvement in patients with COVID-19, hypoxic respiratory failure, and signs of systemic cytokine release syndrome.
Methods We did a prospective, multicentre, open-label, randomised, controlled trial, in hospitalised patients with COVID-19, hypoxia, and signs of a cytokine release syndrome across 16 hospitals in Belgium. Eligible patients had a proven diagnosis of COVID-19 with symptoms between 6 and 16 days, a ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO 2 :FiO 2 ) of less than 350 mm Hg on room air or less than 280 mm Hg on supplemental oxygen, and signs of a cytokine release syndrome in their serum (either a single ferritin measurement of more than 2000 µg/L and immediately requiring high flow oxygen or mechanical ventilation, or a ferritin concentration of more than 1000 µg/L, which had been increasing over the previous 24 h, or lymphopenia below 800/mL with two of the following criteria: an increasing ferritin concentration of more than 700 µg/L, an increasing lactate dehydrogenase concentration of more than 300 international units per L, an increasing C-reactive protein concentration of more than 70 mg/L, or an increasing D-dimers concentration of more than 1000 ng/mL). The COV-AID trial has a 2 × 2 factorial design to evaluate IL-1 blockade versus no IL-1 blockade and IL-6 blockade versus no IL-6 blockade. Patients were randomly assigned by means of permuted block randomisation with varying block size and stratification by centre. In a first randomisation, patients were assigned to receive subcutaneous anakinra once daily (100 mg) for 28 days or until discharge, or to receive no IL-1 blockade (1:2). In a second randomisation step, patients were allocated to receive a single dose of siltuximab (11 mg/kg) intravenously, or a single dose of tocilizumab (8 mg/kg) intravenously, or to receive no IL-6 blockade (1:1:1). The primary outcome was the time to clinical improvement, defined as time from randomisation to an increase of at least two points on a 6-category ordinal scale or to discharge from hospital alive. The primary and supportive efficacy endpoints were assessed in the intention-to-treat population. Safety was assessed in the safety population. This study is registered online with ClinicalTrials.gov (NCT04330638) and EudraCT (2020-001500-41) and is complete. Findings Between April 4, and Dec 6, 2020, 342 patients were randomly assigned to IL-1 blockade (n=112) or no IL-1 blockade (n=230) and simultaneously randomly assigned to IL-6 blockade (n=227; 114 for tocilizumab and 113 for siltuximab) or no IL-6 blockade (n=115). Most patients were male (265 [77%] of 342), median age was..
Causes of death COVID Acute kidney injury
Data sharing De-identified individual participant data will be available on approval of a proposal. The shared data can be used for the analyses mentioned in the approved proposal. The study protocol has been published before. 33 Proposals should be directed to the corresponding author.
References
Abers, Delmonte, Ricotta, An immune-based biomarker signature is associated with mortality in COVID-19 patients, JCI Insight
Angriman, Ferreyro, Burry, Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context, Lancet Respir Med
Aziz, Fatima, Assaly, Elevated interleukin-6 and severe COVID-19: a meta-analysis, J Med Virol
Cavalli, Luca, Campochiaro, Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheumatol
Collignon, Burman, Posch, Schiel, Collaborative platform trials to fight COVID-19: methodological and regulatory considerations for a better societal outcome, Clin Pharmacol Ther
Dodd, Freidlin, Korn, Platform trials-beware the noncomparable control group, N Engl J Med
Galván-Román, Sc, Vallejo, IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study, J Allergy Clin Immunol
Gordon, Mouncey, Al-Beidh, Interleukin-6 receptor antagonists in critically ill patients with Covid-19, N Engl J Med
Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Inform
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hermine, Tharaux, Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial, JAMA Intern Med
Herold, Jurinovic, Arnreich, Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J Allergy Clin Immunol
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with covid-19, N Engl J Med
Horby, Pessoa-Amorim, Peto, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Jones, Hunter, Is IL-6 a key cytokine target for therapy in COVID-19?, Nat Rev Immunol
Kyriazopoulou, Poulakou, Milionis, Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial, Nat Med, doi:10.1038/s41591-021-01499-z
Leisman, Ronner, Pinotti, Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes, Lancet Respir Med
Lescure, Honda, Fowler, Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial, Lancet Respir Med
Maes, Bosteels, Leeuw, Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial, Trials
Matthay, Luetkemeyer, IL-6 receptor antagonist therapy for patients hospitalized for COVID-19: who, when, and how?, JAMA
Mcelvaney, Mcevoy, Mcelvaney, Characterization of the Inflammatory Response to Severe COVID-19 Illness, Am J Respir Crit Care Med
Nagant, Ponthieux, Smet, A score combining early detection of cytokines accurately predicts COVID-19 severity and intensive care unit transfer, Int J Infect Dis
Netea, Rovina, Complex immune dysregulation in covid-19 patients with severe respiratory failure, Cell Host Microbe
Rockwood, Song, Macknight, A global clinical measure of fitness and frailty in elderly people, CMAJ
Rosas, Bräu, Waters, Tocilizumab in hospitalized patients with severe Covid-19 pneumonia, N Engl J Med
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Salvarani, Dolci, Massari, Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial, JAMA Intern Med
Shankar-Hari, Vale, Godolphin, Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis, JAMA
Sinha, Matthay, Calfee, Is a "Cytokine storm" relevant to COVID-19?, JAMA Intern Med
Soin, Kumar, Choudhary, Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial, Lancet Respir Med
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: meta-analysis A, JAMA
Stone, Frigault, Nj, Efficacy of tocilizumab in patients hospitalized with Covid-19, N Engl J Med
Tharaux, Pialoux, Pavot, Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-tomoderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial, Lancet Respir Med
Valle, Kim-Schulze, Huang, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med
Veiga, Prats, Farias, Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial, BMJ
Wang, Fu, Peng, Tocilizumab in patients with moderate or severe COVID-19: a randomized, controlled, open-label, multicenter trial, Front Med
Wilson, Calfee, ARDS Subphenotypes: understanding a heterogeneous syndrome, Crit Care
Zhu, Pang, Ji, Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis, J Med Virol
DOI record:
{
"DOI": "10.1016/s2213-2600(21)00377-5",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(21)00377-5",
"alternative-id": [
"S2213260021003775"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(21)00377-5"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(21)00405-7"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Declercq",
"given": "Jozefien",
"sequence": "first"
},
{
"affiliation": [],
"family": "Van Damme",
"given": "Karel F A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Leeuw",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maes",
"given": "Bastiaan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bosteels",
"given": "Cedric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tavernier",
"given": "Simon J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Buyser",
"given": "Stefanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colman",
"given": "Roos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hites",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verschelden",
"given": "Gil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fivez",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moerman",
"given": "Filip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Demedts",
"given": "Ingel K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dauby",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Schryver",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Govaerts",
"given": "Elke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vandecasteele",
"given": "Stefaan J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van Laethem",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anguille",
"given": "Sebastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van der Hilst",
"given": "Jeroen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Misset",
"given": "Benoit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slabbynck",
"given": "Hans",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wittebole",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liénart",
"given": "Fabienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Legrand",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buyse",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stevens",
"given": "Dieter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bauters",
"given": "Fre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seys",
"given": "Leen J M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aegerter",
"given": "Helena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smole",
"given": "Ursula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bosteels",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoste",
"given": "Levi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naesens",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haerynck",
"given": "Filomeen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vandekerckhove",
"given": "Linos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Depuydt",
"given": "Pieter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Braeckel",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rottey",
"given": "Sylvie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peene",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van Der Straeten",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hulstaert",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lambrecht",
"given": "Bart N",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
29
]
],
"date-time": "2021-10-29T22:59:49Z",
"timestamp": 1635548389000
},
"deposited": {
"date-parts": [
[
2023,
5,
2
]
],
"date-time": "2023-05-02T06:44:32Z",
"timestamp": 1683009872000
},
"funder": [
{
"DOI": "10.13039/501100004727",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004727",
"id-type": "DOI"
}
],
"name": "VIB"
},
{
"DOI": "10.13039/501100019353",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100019353",
"id-type": "DOI"
}
],
"name": "KCE"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
25
]
],
"date-time": "2024-08-25T14:04:53Z",
"timestamp": 1724594693774
},
"is-referenced-by-count": 94,
"issue": "12",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021003775?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021003775?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1427-1438",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S2213-2600(20)30404-5",
"article-title": "Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes",
"author": "Leisman",
"doi-asserted-by": "crossref",
"first-page": "1233",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00377-5_bib1",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"article-title": "Complex immune dysregulation in covid-19 patients with severe respiratory failure",
"author": "Giamarellos-Bourboulis",
"doi-asserted-by": "crossref",
"first-page": "992",
"journal-title": "Cell Host Microbe",
"key": "10.1016/S2213-2600(21)00377-5_bib2",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.05.008",
"article-title": "Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19",
"author": "Herold",
"doi-asserted-by": "crossref",
"first-page": "128",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/S2213-2600(21)00377-5_bib3",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"article-title": "An inflammatory cytokine signature predicts COVID-19 severity and survival",
"author": "Del Valle",
"doi-asserted-by": "crossref",
"first-page": "1636",
"journal-title": "Nat Med",
"key": "10.1016/S2213-2600(21)00377-5_bib4",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25948",
"article-title": "Elevated interleukin-6 and severe COVID-19: a meta-analysis",
"author": "Aziz",
"doi-asserted-by": "crossref",
"first-page": "2283",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(21)00377-5_bib5",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26085",
"article-title": "Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(21)00377-5_bib6",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1172/jci.insight.144455",
"article-title": "An immune-based biomarker signature is associated with mortality in COVID-19 patients",
"author": "Abers",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight",
"key": "10.1016/S2213-2600(21)00377-5_bib7",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.10.003",
"article-title": "A score combining early detection of cytokines accurately predicts COVID-19 severity and intensive care unit transfer",
"author": "Nagant",
"doi-asserted-by": "crossref",
"first-page": "342",
"journal-title": "Int J Infect Dis",
"key": "10.1016/S2213-2600(21)00377-5_bib8",
"volume": "101",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202005-1583OC",
"article-title": "Characterization of the Inflammatory Response to Severe COVID-19 Illness",
"author": "McElvaney",
"doi-asserted-by": "crossref",
"first-page": "812",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/S2213-2600(21)00377-5_bib9",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00377-5_bib10",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: meta-analysis A",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00377-5_bib11",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(21)00139-9",
"article-title": "Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context",
"author": "Angriman",
"doi-asserted-by": "crossref",
"first-page": "655",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00377-5_bib12",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41577-021-00553-8",
"article-title": "Is IL-6 a key cytokine target for therapy in COVID-19?",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "337",
"journal-title": "Nat Rev Immunol",
"key": "10.1016/S2213-2600(21)00377-5_bib13",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.09.018",
"article-title": "IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study",
"author": "Galván-Román",
"doi-asserted-by": "crossref",
"first-page": "72",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/S2213-2600(21)00377-5_bib14",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.11121",
"article-title": "IL-6 receptor antagonist therapy for patients hospitalized for COVID-19: who, when, and how?",
"author": "Matthay",
"doi-asserted-by": "crossref",
"first-page": "483",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00377-5_bib15",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.3313",
"article-title": "Is a “Cytokine storm” relevant to COVID-19?",
"author": "Sinha",
"doi-asserted-by": "crossref",
"first-page": "1152",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S2213-2600(21)00377-5_bib16",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30556-7",
"article-title": "Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial",
"author": "Tharaux",
"doi-asserted-by": "crossref",
"first-page": "295",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00377-5_bib17",
"volume": "9",
"year": "2021"
},
{
"article-title": "Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial",
"author": "Kyriazopoulou",
"journal-title": "Nat Med",
"key": "10.1016/S2213-2600(21)00377-5_bib18",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028836",
"article-title": "Efficacy of tocilizumab in patients hospitalized with Covid-19",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "2333",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00377-5_bib19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.6820",
"article-title": "Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial",
"author": "Hermine",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S2213-2600(21)00377-5_bib20",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028700",
"article-title": "Tocilizumab in hospitalized patients with severe Covid-19 pneumonia",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1503",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00377-5_bib21",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00081-3",
"article-title": "Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial",
"author": "Soin",
"doi-asserted-by": "crossref",
"first-page": "511",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00377-5_bib22",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n84",
"article-title": "Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial",
"author": "Veiga",
"doi-asserted-by": "crossref",
"first-page": "n84",
"journal-title": "BMJ",
"key": "10.1016/S2213-2600(21)00377-5_bib23",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00099-0",
"article-title": "Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial",
"author": "Lescure",
"doi-asserted-by": "crossref",
"first-page": "522",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00377-5_bib24",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2030340",
"article-title": "Tocilizumab in patients hospitalized with Covid-19 pneumonia",
"author": "Salama",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00377-5_bib25",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with Covid-19",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "1491",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00377-5_bib26",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1007/s11684-020-0824-3",
"article-title": "Tocilizumab in patients with moderate or severe COVID-19: a randomized, controlled, open-label, multicenter trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "486",
"journal-title": "Front Med",
"key": "10.1016/S2213-2600(21)00377-5_bib27",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.6615",
"article-title": "Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial",
"author": "Salvarani",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S2213-2600(21)00377-5_bib28",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00377-5_bib29",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.11330",
"article-title": "Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis",
"author": "Shankar-Hari",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00377-5_bib30",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2102446",
"article-title": "Platform trials—beware the noncomparable control group",
"author": "Dodd",
"doi-asserted-by": "crossref",
"first-page": "1572",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00377-5_bib31",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1503/cmaj.050051",
"article-title": "A global clinical measure of fitness and frailty in elderly people",
"author": "Rockwood",
"doi-asserted-by": "crossref",
"first-page": "489",
"journal-title": "CMAJ",
"key": "10.1016/S2213-2600(21)00377-5_bib32",
"volume": "173",
"year": "2005"
},
{
"DOI": "10.1186/s13063-020-04453-5",
"article-title": "Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial",
"author": "Maes",
"doi-asserted-by": "crossref",
"first-page": "468",
"journal-title": "Trials",
"key": "10.1016/S2213-2600(21)00377-5_bib33",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: building an international community of software platform partners",
"author": "Harris",
"doi-asserted-by": "crossref",
"journal-title": "J Biomed Inform",
"key": "10.1016/S2213-2600(21)00377-5_bib34",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "J Biomed Inform",
"key": "10.1016/S2213-2600(21)00377-5_bib35",
"volume": "42",
"year": "2009"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"first-page": "e325",
"journal-title": "Lancet Rheumatol",
"key": "10.1016/S2213-2600(21)00377-5_bib36",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1002/cpt.2183",
"article-title": "Collaborative platform trials to fight COVID-19: methodological and regulatory considerations for a better societal outcome",
"author": "Collignon",
"doi-asserted-by": "crossref",
"first-page": "311",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/S2213-2600(21)00377-5_bib37",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-2778-x",
"article-title": "ARDS Subphenotypes: understanding a heterogeneous syndrome",
"author": "Wilson",
"doi-asserted-by": "crossref",
"first-page": "102",
"journal-title": "Crit Care",
"key": "10.1016/S2213-2600(21)00377-5_bib38",
"volume": "24",
"year": "2020"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260021003775"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}