Tocilizumab use in patients with moderate to severe COVID‐19: A retrospective cohort study
et al., Journal of Clinical Pharmacy and Therapeutics, doi:10.1111/jcpt.13303, Oct 2020
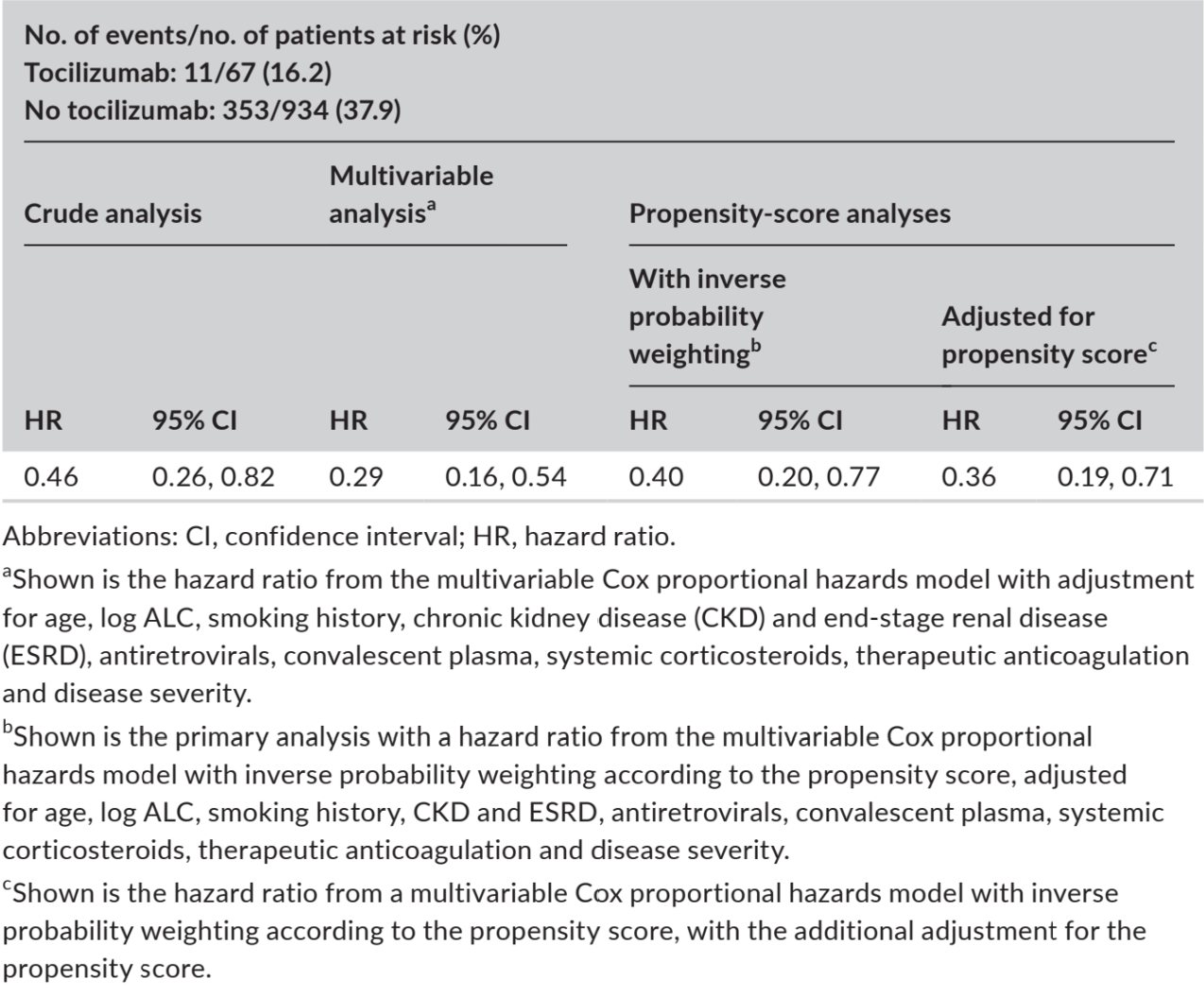
Retrospective 1,225 hospitalized patients showing lower mortality/intubation with tocilizumab.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death/intubation, 60.0% lower, HR 0.40, p = 0.008, treatment 83, control 685, adjusted per study, propensity score weighting, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chilimuri et al., 24 Oct 2020, retrospective, USA, peer-reviewed, 12 authors.
DOI record:
{
"DOI": "10.1111/jcpt.13303",
"ISSN": [
"0269-4727",
"1365-2710"
],
"URL": "http://dx.doi.org/10.1111/jcpt.13303",
"alternative-id": [
"10.1111/jcpt.13303"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-08-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-10-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-10-24"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Chilimuri",
"given": "Sridhar",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3625-2870",
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"authenticated-orcid": false,
"family": "Sun",
"given": "Haozhe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Alemam",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Kang",
"given": "Kyoung‐Sil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Lao",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Mantri",
"given": "Nikhitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Schiller",
"given": "Lawrence",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Sharabun",
"given": "Myroslava",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Shehi",
"given": "Elona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Tejada",
"given": "Jairo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"family": "Yugay",
"given": "Alla",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7829-4024",
"affiliation": [
{
"name": "Department of Medicine Bronxcare Health SystemAffiliated with Icahn School of Medicine at Mount Sinai Bronx NY USA"
}
],
"authenticated-orcid": false,
"family": "Nayudu",
"given": "Suresh Kumar",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Pharmacy and Therapeutics",
"container-title-short": "J Clin Pharm Ther",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
10,
24
]
],
"date-time": "2020-10-24T08:42:05Z",
"timestamp": 1603528925000
},
"deposited": {
"date-parts": [
[
2022,
12,
23
]
],
"date-time": "2022-12-23T18:05:47Z",
"timestamp": 1671818747000
},
"indexed": {
"date-parts": [
[
2025,
5,
14
]
],
"date-time": "2025-05-14T01:24:58Z",
"timestamp": 1747185898174,
"version": "3.40.5"
},
"is-referenced-by-count": 9,
"issue": "2",
"issued": {
"date-parts": [
[
2020,
10,
24
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
24
]
],
"date-time": "2020-10-24T00:00:00Z",
"timestamp": 1603497600000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
24
]
],
"date-time": "2020-10-24T00:00:00Z",
"timestamp": 1603497600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcpt.13303",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/jcpt.13303",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcpt.13303",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "440-446",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2020,
10,
24
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
24
]
]
},
"published-print": {
"date-parts": [
[
2021,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.24171/j.phrp.2020.11.2.03",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_2_1"
},
{
"DOI": "10.1007/s00134-020-06083-6",
"article-title": "Stages or phenotypes? A critical look at COVID‐19 pathophysiology",
"author": "Jain A",
"doi-asserted-by": "crossref",
"first-page": "1494",
"issue": "7",
"journal-title": "Intensive Care Med",
"key": "e_1_2_8_3_1",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06088-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_4_1"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_5_1"
},
{
"DOI": "10.1172/JCI137647",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_6_1"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_7_1"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_8_1"
},
{
"DOI": "10.1002/jmv.25900",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_9_1"
},
{
"DOI": "10.1016/j.cytox.2020.100029",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_10_1"
},
{
"DOI": "10.3949/ccjm.87a.ccc018",
"article-title": "Practical aspects of targeting IL‐6 in COVID‐19 disease",
"author": "Calabrese C",
"doi-asserted-by": "crossref",
"journal-title": "Cleve Clin J Med",
"key": "e_1_2_8_11_1",
"year": "2020"
},
{
"key": "e_1_2_8_12_1",
"unstructured": "FDA.FDA approves tisagenlecleucel for B‐cell ALL and tocilizumab for cytokine release syndrome.https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐approves‐tisagenlecleucel‐b‐cell‐all‐and‐tocilizumab‐cytokine‐release‐syndrome. Accessed May 30 2020."
},
{
"DOI": "10.1016/j.jcv.2020.104443",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_13_1"
},
{
"key": "e_1_2_8_14_1",
"unstructured": "Clinicaltrials.gov.CORIMUNO‐19 ‐ Tocilizumab Trial ‐ TOCI (CORIMUNO‐TOCI) (CORIMUNO‐TOC).https://Clinicaltrials.gov.ct2/show/NCT04331808. Accessed August 1 2020."
},
{
"key": "e_1_2_8_15_1",
"unstructured": "Greater Paris University Hospitals A‐H.Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID‐19 pneumonia.https://www.aphp.fr/contenu/tocilizumab‐improves‐significantly‐clinical‐outcomes‐patients‐moderate‐or‐severe‐covid‐19. Published 2020. Accessed October 15 2020."
},
{
"DOI": "10.1073/pnas.2005615117",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_16_1"
},
{
"key": "e_1_2_8_17_1",
"unstructured": "SAS software. Copyright © [2020] SAS Institute Inc.SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. Cary NC USA."
},
{
"DOI": "10.1080/00273171.2011.568786",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_18_1"
},
{
"key": "e_1_2_8_19_1",
"unstructured": "Clinicaltrials.gov.https://clinicaltrials.gov/ct2/results?cond=Covid‐19&term=tocilizumab&cntry=&state=&city=&dist. Accessed May 30 2020."
},
{
"key": "e_1_2_8_20_1",
"unstructured": "Clinicaltrials.gov.https://Clinicaltrials.gov.ct2/results?cond=COVID&term=sarilumab&cntry=&state=&city=&dist. Accessed May 30 2020."
},
{
"key": "e_1_2_8_21_1",
"unstructured": "TarrytownNY ParisX.Regeneron and sanofi provide update on U.S. phase 2/3 adaptive‐designed trial of Kevzara® (Sarilumab) in hospitalized COVID‐19 patients. 2020.www.fda.gov/medwatch. Accessed May 15 2020."
},
{
"DOI": "10.1111/jdv.16620",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_22_1"
},
{
"DOI": "10.1093/rheumatology/keq343",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_23_1"
},
{
"key": "e_1_2_8_24_1",
"unstructured": "COVID‐19 Treatment Guidelines Panel.Coronavirus disease 2019 (COVID‐19) treatment guidelines. National institutes of health. http://COVID-19TreatmentGuidelinesPanelhttps://www.covid19treatmentguidelines.nih.gov/. Accessed May 30 2020."
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jcpt.13303"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tocilizumab use in patients with moderate to severe COVID‐19: A retrospective cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "46"
}