Effectiveness of molnupiravir and nirmatrelvir–ritonavir in CKD patients with COVID-19
et al., Kidney International Reports, doi:10.1016/j.ekir.2024.02.009, Feb 2024
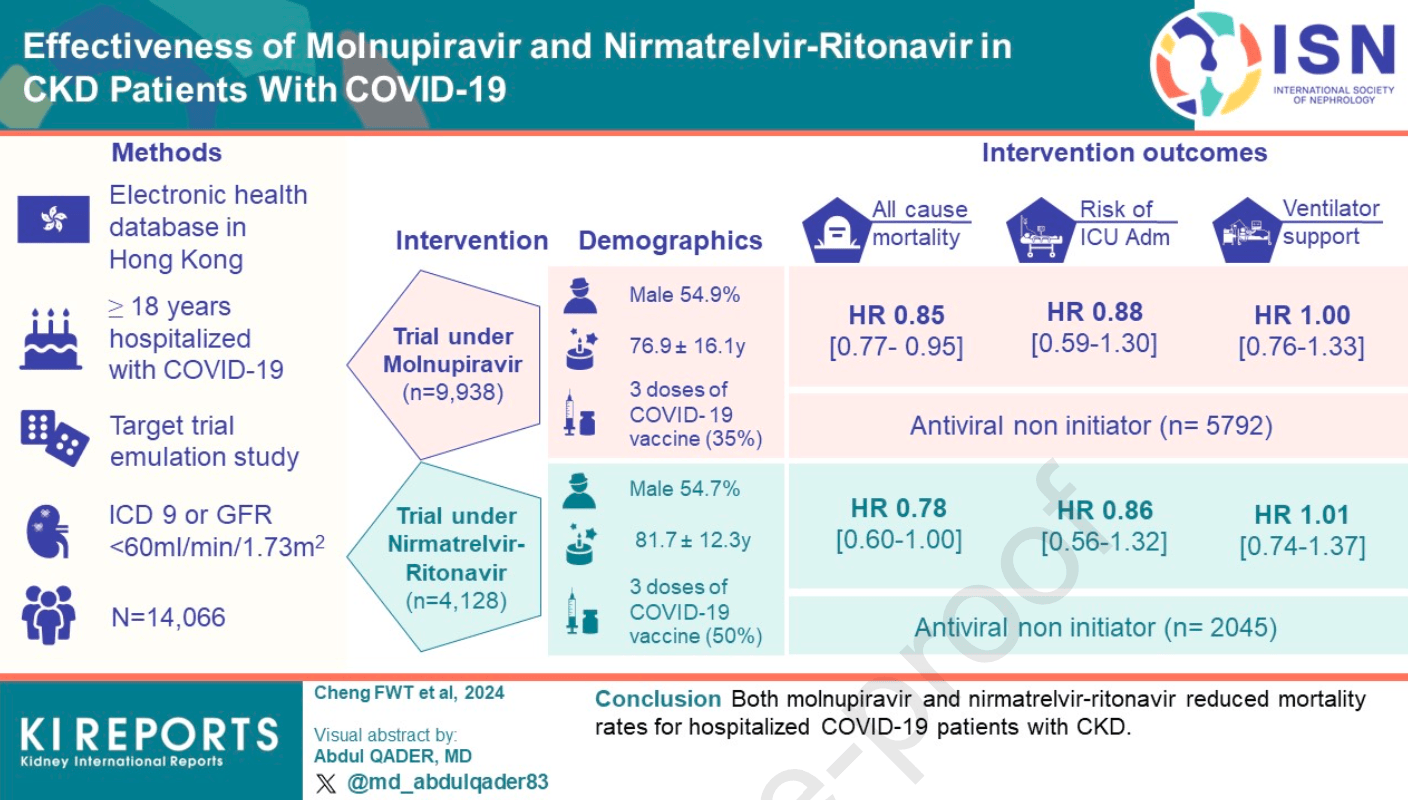
Retrospective emulated target trial of hospitalized COVID-19 patients with chronic kidney disease in Hong Kong showing lower mortality with molnupiravir and paxlovid treatment. No significant reduction was found in ICU admission or ventilatory support.
Notably, there is no estimated benefit for ventilatory support, the outcome most directly related to severe COVID-19.
The very large difference in vaccine uptake suggests major confounding issues, for example treatment decisions based on vaccine uptake, or confounding by time where control patients are more likely from earlier periods with lower vaccine uptake, which may also correspond to more dangerous variants.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
Study covers molnupiravir and paxlovid.
|
risk of death, 22.4% lower, HR 0.78, p = 0.049, treatment 2,083, control 2,045.
|
|
ventilatory support, 0.8% higher, HR 1.01, p = 0.96, treatment 2,083, control 2,045.
|
|
risk of ICU admission, 14.1% lower, HR 0.86, p = 0.50, treatment 2,083, control 2,045.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Cheng et al., 9 Feb 2024, retrospective, China, peer-reviewed, 11 authors.
Contact: ewchan@hku.hk, wongick@hku.hk.
Effectiveness of molnupiravir and nirmatrelvir–ritonavir in CKD patients with COVID-19.
Kidney International Reports, doi:10.1016/j.ekir.2024.02.009
Introduction Even with effective vaccines, patients with CKD have a higher risk of hospitalization and death subsequent to COVID-19 infection compared with those without CKD. Molnupiravir and nirmatrelvir-ritonavir have been approved for emergency use, but their effectiveness for the CKD population is still unknown. This study was conducted to determine the effectiveness of these drugs in reducing mortality and severe COVID-19 in the CKD population. Methods This was a target trial emulation study using electronic health databases in Hong Kong. Patients with CKD aged 18 years or older who were hospitalized with COVID-19 were included. The per-protocol average treatment effect among COVID-19 oral antiviral initiators, including all-cause mortality, ICU admission, and ventilatory support within 28 days, were compared to non-initiators.
Results Antivirals have been found to lower the risk of all-cause mortality, with molnupiravir at a hazard ratio (HR) of 0.85 [95% CI, 0.77 to 0.95] and nirmatrelvir-ritonavir at an HR of 0.78 [CI, 0.60 to 1.00]. However, they do not significantly reduce the risk of ICU admission (molnupiravir: HR, 0.88 [CI, 0.59 to 1.30]; nirmatrelvir-ritonavir: HR, 0.86 [CI, 0.56 to 1.32]) or ventilatory support (molnupiravir: HR, 1.00 [CI, 0.76 to 1.33]; nirmatrelvir-ritonavir: HR, 1.01 [CI, 0.74 to 1.37]). There was a greater risk reduction in males and those with higher Charlson Comorbidity Index. The nirmatrelvir-ritonavir trial also showed reduced risk for those who had antiviral treatment and received three or more vaccine doses.
Conclusion Both molnupiravir and nirmatrelvir-ritonavir reduced mortality rates for hospitalized COVID-19 patients with CKD.
Supplementary Material STROBE statement (PDF) Supplementary Table S1 Target trial specification and emulation using observational data (PDF) Supplementary Table S2 . Baseline and post-assignment covariates for the construction of inverse probability weights (PDF) Supplementary Table S3 . Survival Probability for Outcomes in COVID-19 Oral Antiviral Initiators Compared With Non-initiators (PDF) Supplementary information is available at KI Report's website. J o u r n a l P r e -p r o o f
References
Agostino, Lee, Belanger, Cupples, Anderson et al., Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study, Stat Med
Arribas, Bhagani, Lobo, Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19, NEJM Evidence
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Carr, Kronbichler, Graham-Brown, Review of Early Immune Response to SARS-CoV-2 Vaccination Among Patients With CKD, Kidney Int Rep
Cheng, Fan, Wong, The effectiveness and safety of mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines among individuals with chronic kidney diseases, Kidney Int
Cheng, Wong, Qin, Risk of glomerular diseases, proteinuria and hematuria following mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines, Nephrol Dial Transplant
Cho, Harden, Moreno, Oral antiviral therapies for COVID-19 in patients with advanced chronic kidney disease or kidney failure, Nephrol Dial Transplant
Chua, Kwan, Chui, Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination, Clin Infect Dis
Chui, Wan, Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study
Flythe, Assimon, Tugman, Characteristics and Outcomes of Individuals With Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States, Am J Kidney Dis
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Harrison, Fazio-Eynullayeva, Lane, Underhill, Lip, Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis, PLoS medicine
Hernán, Brumback, Robins, Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men, Epidemiology
Hernán, Methods of Public Health Research -Strengthening Causal Inference from Observational Data, New England Journal of Medicine
Hernán, Robins, Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available, Am J Epidemiol
J O U R N A L P R E, None
Kale, Shelke, Dagar, Anders, Gaikwad, How to use COVID-19 antiviral drugs in patients with chronic kidney disease, Front Pharmacol
Khan, Khan, Mustagir, Rana, Islam et al., Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis, J Glob Health
Kragholm, Andersen, Gerds, Association Between Male Sex and Outcomes of Coronavirus Disease 2019 (COVID-19)-A Danish Nationwide, Registerbased Study, Clin Infect Dis
Lai, Li, Peng, Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine : A Case-Control Study, Ann Intern Med
Lawrence, Mirchandani, Hill, Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13,694 patients
Peckham, De Gruijter, Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission, Nat Commun
Petrilli, Jones, Yang, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
Taji, Thomas, Oliver, COVID-19 in patients undergoing long-term dialysis in Ontario, CMAJ
Toussi, Neutel, Navarro, Pharmacokinetics of Oral Nirmatrelvir/Ritonavir, a Protease Inhibitor for Treatment of COVID-19, in Subjects With Renal Impairment, Clin Pharmacol Ther
Wan, Chui, Lai, Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study, Lancet Infect Dis
Wan, Chui, Wang, Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: A self-controlled case series and nested case-control study, Lancet Reg Health West Pac
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature
Wong, Lau, Xiong, Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: A retrospective study, PLoS Med
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, The lancet
DOI record:
{
"DOI": "10.1016/j.ekir.2024.02.009",
"ISSN": [
"2468-0249"
],
"URL": "http://dx.doi.org/10.1016/j.ekir.2024.02.009",
"alternative-id": [
"S2468024924000986"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effectiveness of molnupiravir and nirmatrelvir–ritonavir in CKD patients with COVID-19."
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Kidney International Reports"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ekir.2024.02.009"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Published by Elsevier Inc. on behalf of the International Society of Nephrology."
}
],
"author": [
{
"affiliation": [],
"family": "Cheng",
"given": "Franco Wing Tak",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yan",
"given": "Vincent Ka Chun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wan",
"given": "Eric Yuk Fai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chui",
"given": "Celine Sze Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lai",
"given": "Francisco Tsz Tsun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Carlos King Ho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Irene Ran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Sydney Chi Wai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Ian Chi Kei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chan",
"given": "Esther Wai Yin",
"sequence": "additional"
}
],
"container-title": "Kidney International Reports",
"container-title-short": "Kidney International Reports",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"kireports.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T17:40:16Z",
"timestamp": 1707500416000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T17:40:32Z",
"timestamp": 1707500432000
},
"indexed": {
"date-parts": [
[
2024,
2,
11
]
],
"date-time": "2024-02-11T12:16:36Z",
"timestamp": 1707653796441
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 5,
"start": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T00:00:00Z",
"timestamp": 1707177600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2468024924000986?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2468024924000986?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
2
]
]
},
"published-print": {
"date-parts": [
[
2024,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "Factors associated with COVID-19-related death using OpenSAFELY",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Nature",
"key": "10.1016/j.ekir.2024.02.009_bib1",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.7189/jogh.10.020503",
"article-title": "Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis",
"author": "Khan",
"doi-asserted-by": "crossref",
"journal-title": "J Glob Health",
"key": "10.1016/j.ekir.2024.02.009_bib2",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1053/j.ajkd.2020.09.003",
"article-title": "Characteristics and Outcomes of Individuals With Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States",
"author": "Flythe",
"doi-asserted-by": "crossref",
"first-page": "190",
"journal-title": "Am J Kidney Dis",
"key": "10.1016/j.ekir.2024.02.009_bib3",
"volume": "77",
"year": "2021"
},
{
"article-title": "The effectiveness and safety of mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines among individuals with chronic kidney diseases",
"author": "Cheng",
"issue": "22",
"journal-title": "Kidney Int",
"key": "10.1016/j.ekir.2024.02.009_bib4",
"volume": "S0085-2538",
"year": "2022"
},
{
"DOI": "10.1503/cmaj.202601",
"article-title": "COVID-19 in patients undergoing long-term dialysis in Ontario",
"author": "Taji",
"doi-asserted-by": "crossref",
"first-page": "E278",
"journal-title": "CMAJ",
"key": "10.1016/j.ekir.2024.02.009_bib5",
"volume": "193",
"year": "2021"
},
{
"DOI": "10.1016/j.ekir.2021.06.027",
"article-title": "Review of Early Immune Response to SARS-CoV-2 Vaccination Among Patients With CKD",
"author": "Carr",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "Kidney Int Rep",
"key": "10.1016/j.ekir.2024.02.009_bib6",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "10.1016/j.ekir.2024.02.009_bib7",
"volume": "602",
"year": "2022"
},
{
"key": "10.1016/j.ekir.2024.02.009_bib8",
"unstructured": "Update: FDA Authorizes Additional Oral Antiviral Treatment of COVID-19 in Certain Adults. 2021;"
},
{
"key": "10.1016/j.ekir.2024.02.009_bib9",
"unstructured": "Update: FDA authorizes first oral antiviral for treatment of COVID-19. 2021;"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ekir.2024.02.009_bib10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ekir.2024.02.009_bib11",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2023.1053814",
"article-title": "How to use COVID-19 antiviral drugs in patients with chronic kidney disease",
"author": "Kale",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ekir.2024.02.009_bib12",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1002/cpt.2688",
"article-title": "Pharmacokinetics of Oral Nirmatrelvir/Ritonavir, a Protease Inhibitor for Treatment of COVID-19, in Subjects With Renal Impairment",
"author": "Toussi",
"doi-asserted-by": "crossref",
"first-page": "892",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ekir.2024.02.009_bib13",
"volume": "112",
"year": "2022"
},
{
"key": "10.1016/j.ekir.2024.02.009_bib14",
"unstructured": "Free COVID-19 oral drugs plan set. 2022;"
},
{
"key": "10.1016/j.ekir.2024.02.009_bib15",
"unstructured": "LCQ7: Introduction of new drugs for treating Coronavirus Disease 2019. 2022;"
},
{
"key": "10.1016/j.ekir.2024.02.009_bib16",
"unstructured": "First shipment of COVID-19 oral drug Paxlovid distributed to HA for application (with photos). 2022;"
},
{
"article-title": "Risk of glomerular diseases, proteinuria and hematuria following mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines",
"author": "Cheng",
"journal-title": "Nephrol Dial Transplant",
"key": "10.1016/j.ekir.2024.02.009_bib17",
"year": "2022"
},
{
"article-title": "Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination",
"author": "Chua",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ekir.2024.02.009_bib18",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2022.101504",
"article-title": "Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study",
"author": "Chui",
"doi-asserted-by": "crossref",
"journal-title": "eClinicalMedicine",
"key": "10.1016/j.ekir.2024.02.009_bib19",
"year": "2022"
},
{
"DOI": "10.7326/M21-3700",
"article-title": "Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine : A Case-Control Study",
"author": "Lai",
"doi-asserted-by": "crossref",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.ekir.2024.02.009_bib20",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00451-5",
"article-title": "Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study",
"author": "Wan",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ekir.2024.02.009_bib21",
"volume": "22",
"year": "2022"
},
{
"article-title": "Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: A self-controlled case series and nested case-control study",
"author": "Wan",
"journal-title": "Lancet Reg Health West Pac",
"key": "10.1016/j.ekir.2024.02.009_bib22",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1371/journal.pmed.1004018",
"article-title": "Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: A retrospective study",
"author": "Wong",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Med",
"key": "10.1016/j.ekir.2024.02.009_bib23",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1056/NEJMp2113319",
"article-title": "Methods of Public Health Research — Strengthening Causal Inference from Observational Data",
"author": "Hernán",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.ekir.2024.02.009_bib24",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/aje/kwv254",
"article-title": "Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available",
"author": "Hernán",
"doi-asserted-by": "crossref",
"first-page": "758",
"journal-title": "Am J Epidemiol",
"key": "10.1016/j.ekir.2024.02.009_bib25",
"volume": "183",
"year": "2016"
},
{
"key": "10.1016/j.ekir.2024.02.009_bib26",
"unstructured": "Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) For Paxlovid. 2022;"
},
{
"DOI": "10.1002/sim.4780091214",
"article-title": "Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study",
"author": "D’Agostino",
"doi-asserted-by": "crossref",
"first-page": "1501",
"journal-title": "Stat Med",
"key": "10.1016/j.ekir.2024.02.009_bib27",
"volume": "9",
"year": "1990"
},
{
"DOI": "10.1097/00001648-200009000-00012",
"article-title": "Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men",
"author": "Hernán",
"doi-asserted-by": "crossref",
"first-page": "561",
"journal-title": "Epidemiology",
"key": "10.1016/j.ekir.2024.02.009_bib28",
"volume": "11",
"year": "2000"
},
{
"DOI": "10.1056/EVIDoa2100044",
"article-title": "Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19",
"author": "Arribas",
"doi-asserted-by": "crossref",
"journal-title": "NEJM Evidence",
"key": "10.1016/j.ekir.2024.02.009_bib29",
"volume": "1",
"year": "2022"
},
{
"article-title": "Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13,694 patients",
"author": "Lawrence",
"journal-title": "Research Square",
"key": "10.1016/j.ekir.2024.02.009_bib30",
"year": "2022"
},
{
"article-title": "Oral antiviral therapies for COVID-19 in patients with advanced chronic kidney disease or kidney failure",
"author": "Cho",
"journal-title": "Nephrol Dial Transplant",
"key": "10.1016/j.ekir.2024.02.009_bib31",
"year": "2023"
},
{
"DOI": "10.1371/journal.pmed.1003321",
"article-title": "Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis",
"author": "Harrison",
"doi-asserted-by": "crossref",
"journal-title": "PLoS medicine",
"key": "10.1016/j.ekir.2024.02.009_bib32",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "The lancet",
"key": "10.1016/j.ekir.2024.02.009_bib33",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1966",
"article-title": "Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study",
"author": "Petrilli",
"doi-asserted-by": "crossref",
"first-page": "m1966",
"journal-title": "BMJ",
"key": "10.1016/j.ekir.2024.02.009_bib34",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa924",
"article-title": "Association Between Male Sex and Outcomes of Coronavirus Disease 2019 (COVID-19)-A Danish Nationwide, Register-based Study",
"author": "Kragholm",
"doi-asserted-by": "crossref",
"first-page": "e4025",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ekir.2024.02.009_bib35",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19741-6",
"article-title": "Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission",
"author": "Peckham",
"doi-asserted-by": "crossref",
"first-page": "6317",
"journal-title": "Nat Commun",
"key": "10.1016/j.ekir.2024.02.009_bib36",
"volume": "11",
"year": "2020"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2468024924000986"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nephrology"
],
"subtitle": [],
"title": "Effectiveness of molnupiravir and nirmatrelvir–ritonavir in CKD patients with COVID-19.",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
cheng4