Mitoquinone mesylate as post-exposure prophylaxis against SARS-CoV-2 infection in humans: an exploratory single center pragmatic open label non-randomized pilot clinical trial with matched controls
et al., eBioMedicine, doi:10.1016/j.ebiom.2024.105042, NCT05381454, Mar 2024
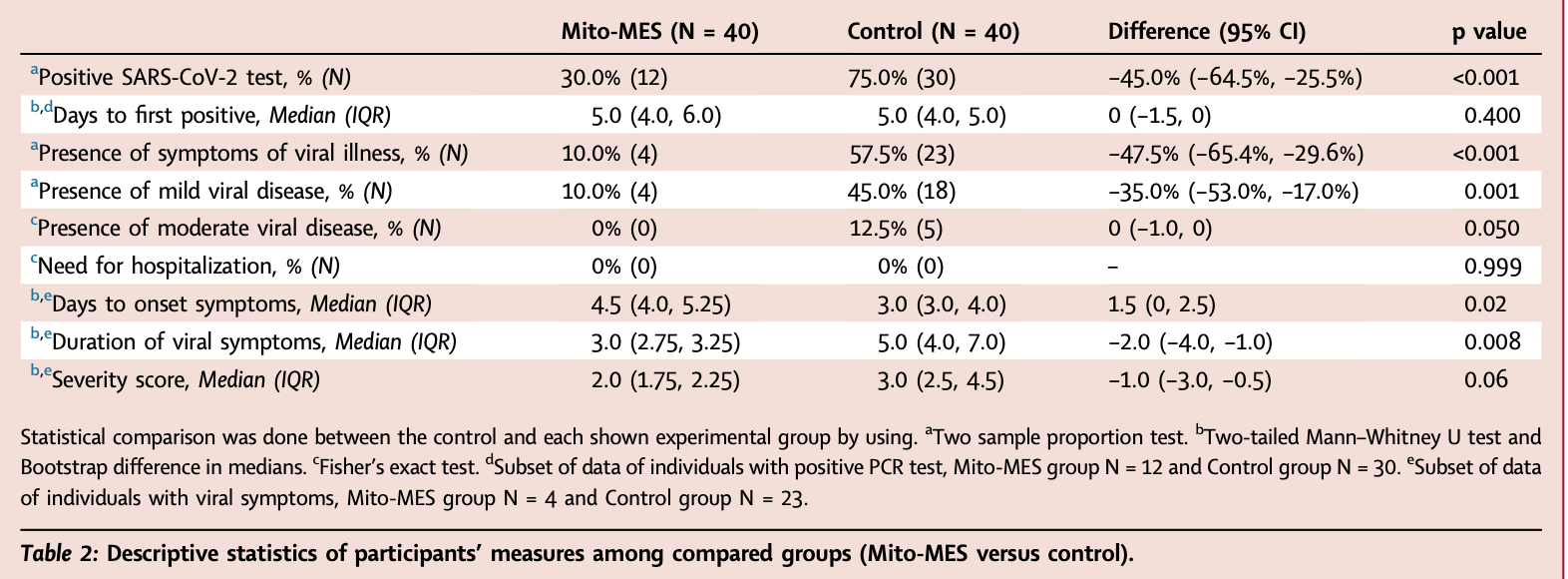
Open-label non-randomized trial with 80 participants exposed to confirmed SARS-CoV-2 cases, showing lower risk of infection and milder symptoms with mitoquinone mesylate (Mito-MES) prophylaxis. 40 participants took Mito-MES 20mg daily for 14 days, starting within 5 days of exposure, while 40 did not take Mito-MES. 30% of Mito-MES participants tested positive for SARS-CoV-2 compared to 75% of controls. None of the participants who started Mito-MES within 72 hours developed infection, compared to 12 who started on days 3-5. There was no hospitalization in either group.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
moderate disease, 90.9% lower, RR 0.09, p = 0.05, treatment 0 of 40 (0.0%), control 5 of 40 (12.5%), NNT 8.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
mild disease, 77.8% lower, RR 0.22, p < 0.001, treatment 4 of 40 (10.0%), control 18 of 40 (45.0%), NNT 2.9.
|
|
risk of symptomatic case, 82.6% lower, RR 0.17, p < 0.001, treatment 4 of 40 (10.0%), control 23 of 40 (57.5%), NNT 2.1.
|
|
risk of case, 60.0% lower, RR 0.40, p < 0.001, treatment 12 of 40 (30.0%), control 30 of 40 (75.0%), NNT 2.2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chen et al., 11 Mar 2024, prospective, USA, peer-reviewed, 3 authors, study period 1 May, 2022 - 1 December, 2022, trial NCT05381454 (history).
Contact: theodoros.kelesidis@utsouthwestern.edu, tkelesidis@g.ucla.edu.
Mitoquinone mesylate as post-exposure prophylaxis against SARS-CoV-2 infection in humans: an exploratory single center pragmatic open label non-randomized pilot clinical trial with matched controls
eBioMedicine, doi:10.1016/j.ebiom.2024.105042
Background An ongoing important need exists to rapidly develop novel therapeutics for COVID-19 that will retain antiviral efficacy in the setting of rapidly evolving SARS-CoV-2 variants and potential future development of resistance of SARS-COV-2 to remdesivir and protease inhibitors. To date, there is no FDA-approved treatment for post-exposure prophylaxis against SAR-CoV-2. We have shown that the mitochondrial antioxidant mitoquinone/ mitoquinol mesylate (Mito-MES), a dietary supplement, has antiviral activity against SARS-CoV-2 in vitro and in SARS-CoV-2 infected K18-hACE2 mice.
Methods In this exploratory, pragmatic open label clinical trial (ClinicalTrials.gov identifier NCT05381454), we studied whether Mito-MES is an effective post-exposure prophylaxis treatment in people who had high-grade unmasked exposures to SARS-CoV-2 within 5 days prior to study entry. Participants were enrolled in real-world setting in Los Angeles, United States between May 1 and December 1, 2022 and were assigned to either mito-MES 20 mg daily for 14 days (n = 40) or no mito-MES (controls) (n = 40). The primary endpoint was development of SARS-CoV-2 infection based on 4 COVID-19 diagnostic tests [rapid antigen tests (RATs) or PCR] performed during the study period (14 days post exposure). Findings Out of 40 (23 females; 57.5%) study participants who took Mito-MES, 12 (30%) developed SARS-CoV-2 infection compared to 30 of the 40 controls (75%) (difference -45.0%, 95% confidence intervals (CI): -64.5%, -25.5%). Out of 40 (19 females; 47.5%) study participants in the control group, 30 (75.0%) had at least one positive COVID-19 diagnostic test and 23 (57.5%) were symptomatic. With regards to key secondary outcomes, among symptomatic SARS-CoV-2 infections, the median duration of viral symptoms was lower in the Mito-MES group (median 3.0, 95% CI 2.75, 3.25) compared to the control group (median 5.0, 95% CI 4.0, 7.0). None of the study participants was hospitalized or required oxygen therapy. Mito-MES was well tolerated and no serious side effect was reported in any study participant. Interpretation This work describes antiviral activity of mito-MES in humans. Mito-MES was well tolerated in our study population and attenuated transmission of SARS-CoV-2 infection. Given established safety of Mito-MES in humans, our results suggest that randomized control clinical trials of Mito-MES as post-exposure prophylaxis against SARS-CoV-2 infection are warranted.
References
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Dashdorj, Jyothi, Lim, Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines, BMC Med
Fujimoto, Kurihara, Hirata, Takeda, Effects of coenzyme Q10 administration on pulmonary function and exercise performance in patients with chronic lung diseases, Clin Investig
Gandhi, Klein, Robertson, De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report, Nat Commun
Gane, Weilert, Orr, The mitochondria-targeted antioxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients, Liver Int
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med
Higgins, Phillpotts, Scott, Wallace, Bernhardt et al., Intranasal interferon as protection against experimental respiratory coronavirus infection in volunteers, Antimicrob Agents Chemother
Hu, Bogoyevitch, Jans, Subversion of host cell mitochondria by RSV to favor virus production is dependent on inhibition of mitochondrial complex I and ROS generation, Cells
Hu, Schulze, Ghildyal, Respiratory syncytial virus coopts host mitochondrial function to favour infectious virus production, Elife
Lother, Abassi, Agostinis, Post-exposure prophylaxis or pre-emptive therapy for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): study protocol for a pragmatic randomized-controlled trial, Can J Anaesth
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (paxlovid) and comedications, Clin Pharmacol Ther
Mcburney, A dose ranging study of the pharmacokinetics and safety of mitoquinone administration in healthy adult volunteers, Huntingdon Life Science Ltd
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med
Patsopoulos, A pragmatic view on pragmatic trials, Dialogues Clin Neurosci
Petcherski, Sharma, Satta, Mitoquinone mesylate targets SARS-CoV-2 infection in preclinical models, bioRxiv, doi:10.1101/2022.02.22.481100
Pham, Macrae, Broome, MitoQ and CoQ10 supplementation mildly suppresses skeletal muscle mitochondrial hydrogen peroxide levels without impacting mitochondrial function in middle-aged men, Eur J Appl Physiol
Ribero, Jouvenet, Dreux, Nisole, Interplay between SARS-CoV-2 and the type I interferon response, PLoS Pathog
Robinson, Mirza, Gallagher, Limitations of molecular and antigen test performance for SARS-CoV-2 in symptomatic and asymptomatic COVID-19 contacts, J Clin Microbiol
Rossman, Santos-Parker, Steward, Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults, Hypertension
Smith, Murphy, Animal and human studies with the mitochondria-targeted antioxidant MitoQ, Ann N Y Acad Sci
Smith, Porteous, Gane, Murphy, Delivery of bioactive molecules to mitochondria in vivo, Proc Natl Acad Sci U S A
Snow, Rolfe, Lockhart, A double-blind, placebocontrolled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease, Mov Disord
Sticher, Lu, Mitchell, Analysis of the potential for N (4)-hydroxycytidine to inhibit mitochondrial replication and function, Antimicrob Agents Chemother
Tan, Chan, Juni, Post-exposure prophylaxis against SARS-CoV-2 in close contacts of confirmed COVID-19 cases (CORIPREV): study protocol for a cluster-randomized trial, Trials
Vandyck, Deval, Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection, Curr Opin Virol
Williamson, Hughes, Cobley, Davison, The mitochondria-targeted antioxidant MitoQ, attenuates exerciseinduced mitochondrial DNA damage, Redox Biol
Zaki, Strategies for oral delivery and mitochondrial targeting of CoQ10, Drug Deliv
Zhou, Hill, Sarkar, beta-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells, J Infect Dis
DOI record:
{
"DOI": "10.1016/j.ebiom.2024.105042",
"ISSN": [
"2352-3964"
],
"URL": "http://dx.doi.org/10.1016/j.ebiom.2024.105042",
"alternative-id": [
"S235239642400077X"
],
"article-number": "105042",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Mitoquinone mesylate as post-exposure prophylaxis against SARS-CoV-2 infection in humans: an exploratory single center pragmatic open label non-randomized pilot clinical trial with matched controls"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eBioMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ebiom.2024.105042"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Chen",
"given": "Keren",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jackson",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8463-3811",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kelesidis",
"given": "Theodoros",
"sequence": "additional"
}
],
"container-title": "eBioMedicine",
"container-title-short": "eBioMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
11
]
],
"date-time": "2024-03-11T23:29:30Z",
"timestamp": 1710199770000
},
"deposited": {
"date-parts": [
[
2024,
3,
11
]
],
"date-time": "2024-03-11T23:29:45Z",
"timestamp": 1710199785000
},
"funder": [
{
"DOI": "10.13039/100000002",
"doi-asserted-by": "publisher",
"name": "NIH"
},
{
"DOI": "10.13039/100005192",
"doi-asserted-by": "publisher",
"name": "California HIV/AIDS Research Program"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
12
]
],
"date-time": "2024-03-12T00:26:08Z",
"timestamp": 1710203168715
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
19
]
],
"date-time": "2024-02-19T00:00:00Z",
"timestamp": 1708300800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S235239642400077X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S235239642400077X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105042",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
3
]
]
},
"published-print": {
"date-parts": [
[
2024,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.coviro.2021.04.006",
"article-title": "Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection",
"author": "Vandyck",
"doi-asserted-by": "crossref",
"first-page": "36",
"journal-title": "Curr Opin Virol",
"key": "10.1016/j.ebiom.2024.105042_bib1",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1128/AAC.01719-19",
"article-title": "Analysis of the potential for N (4)-hydroxycytidine to inhibit mitochondrial replication and function",
"author": "Sticher",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ebiom.2024.105042_bib2",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiab247",
"article-title": "beta-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "415",
"issue": "3",
"journal-title": "J Infect Dis",
"key": "10.1016/j.ebiom.2024.105042_bib3",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (paxlovid) and comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"issue": "6",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ebiom.2024.105042_bib4",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-29104-y",
"article-title": "De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1547",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.ebiom.2024.105042_bib5",
"volume": "13",
"year": "2022"
},
{
"article-title": "Mitoquinone mesylate targets SARS-CoV-2 infection in preclinical models",
"author": "Petcherski",
"journal-title": "bioRxiv",
"key": "10.1016/j.ebiom.2024.105042_bib6",
"year": "2022"
},
{
"DOI": "10.1111/j.1749-6632.2010.05627.x",
"article-title": "Animal and human studies with the mitochondria-targeted antioxidant MitoQ",
"author": "Smith",
"doi-asserted-by": "crossref",
"first-page": "96",
"journal-title": "Ann N Y Acad Sci",
"key": "10.1016/j.ebiom.2024.105042_bib7",
"volume": "1201",
"year": "2010"
},
{
"DOI": "10.1111/j.1478-3231.2010.02250.x",
"article-title": "The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients",
"author": "Gane",
"doi-asserted-by": "crossref",
"first-page": "1019",
"issue": "7",
"journal-title": "Liver Int",
"key": "10.1016/j.ebiom.2024.105042_bib8",
"volume": "30",
"year": "2010"
},
{
"DOI": "10.1002/mds.23148",
"article-title": "A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease",
"author": "Snow",
"doi-asserted-by": "crossref",
"first-page": "1670",
"issue": "11",
"journal-title": "Mov Disord",
"key": "10.1016/j.ebiom.2024.105042_bib9",
"volume": "25",
"year": "2010"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.117.10787",
"article-title": "Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults",
"author": "Rossman",
"doi-asserted-by": "crossref",
"first-page": "1056",
"issue": "6",
"journal-title": "Hypertension",
"key": "10.1016/j.ebiom.2024.105042_bib10",
"volume": "71",
"year": "2018"
},
{
"DOI": "10.1016/j.redox.2020.101673",
"article-title": "The mitochondria-targeted antioxidant MitoQ, attenuates exercise-induced mitochondrial DNA damage",
"author": "Williamson",
"doi-asserted-by": "crossref",
"journal-title": "Redox Biol",
"key": "10.1016/j.ebiom.2024.105042_bib11",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1007/s00421-020-04396-4",
"article-title": "MitoQ and CoQ10 supplementation mildly suppresses skeletal muscle mitochondrial hydrogen peroxide levels without impacting mitochondrial function in middle-aged men",
"author": "Pham",
"doi-asserted-by": "crossref",
"first-page": "1657",
"issue": "7",
"journal-title": "Eur J Appl Physiol",
"key": "10.1016/j.ebiom.2024.105042_bib12",
"volume": "120",
"year": "2020"
},
{
"DOI": "10.7554/eLife.42448",
"article-title": "Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production",
"author": "Hu",
"doi-asserted-by": "crossref",
"journal-title": "Elife",
"key": "10.1016/j.ebiom.2024.105042_bib13",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "657",
"issue": "7898",
"journal-title": "Nature",
"key": "10.1016/j.ebiom.2024.105042_bib14",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1073/pnas.0931245100",
"article-title": "Delivery of bioactive molecules to mitochondria in vivo",
"author": "Smith",
"doi-asserted-by": "crossref",
"first-page": "5407",
"issue": "9",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.ebiom.2024.105042_bib15",
"volume": "100",
"year": "2003"
},
{
"DOI": "10.3390/cells8111417",
"article-title": "Subversion of host cell mitochondria by RSV to favor virus production is dependent on inhibition of mitochondrial complex I and ROS generation",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "1417",
"issue": "11",
"journal-title": "Cells",
"key": "10.1016/j.ebiom.2024.105042_bib16",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1186/1741-7015-11-178",
"article-title": "Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines",
"author": "Dashdorj",
"doi-asserted-by": "crossref",
"first-page": "178",
"journal-title": "BMC Med",
"key": "10.1016/j.ebiom.2024.105042_bib17",
"volume": "11",
"year": "2013"
},
{
"DOI": "10.1007/s12630-020-01684-7",
"article-title": "Post-exposure prophylaxis or pre-emptive therapy for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): study protocol for a pragmatic randomized-controlled trial",
"author": "Lother",
"doi-asserted-by": "crossref",
"first-page": "1201",
"issue": "9",
"journal-title": "Can J Anaesth",
"key": "10.1016/j.ebiom.2024.105042_bib18",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05134-7",
"article-title": "Post-exposure prophylaxis against SARS-CoV-2 in close contacts of confirmed COVID-19 cases (CORIPREV): study protocol for a cluster-randomized trial",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "224",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.ebiom.2024.105042_bib19",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ebiom.2024.105042_bib20",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/j.ebiom.2024.105042_bib21",
"series-title": "Assessing COVID-19-related symptoms in outpatient adult and adolescent Subjects in clinical trials of drugs and biological Products for COVID-19 prevention or treatment Guidance for industry",
"year": "2020"
},
{
"DOI": "10.1128/jcm.00187-22",
"article-title": "Limitations of molecular and antigen test performance for SARS-CoV-2 in symptomatic and asymptomatic COVID-19 contacts",
"author": "Robinson",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "J Clin Microbiol",
"key": "10.1016/j.ebiom.2024.105042_bib22",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.31887/DCNS.2011.13.2/npatsopoulos",
"article-title": "A pragmatic view on pragmatic trials",
"author": "Patsopoulos",
"doi-asserted-by": "crossref",
"first-page": "217",
"issue": "2",
"journal-title": "Dialogues Clin Neurosci",
"key": "10.1016/j.ebiom.2024.105042_bib23",
"volume": "13",
"year": "2011"
},
{
"article-title": "Effects of coenzyme Q10 administration on pulmonary function and exercise performance in patients with chronic lung diseases",
"author": "Fujimoto",
"first-page": "S162",
"issue": "8 Suppl",
"journal-title": "Clin Investig",
"key": "10.1016/j.ebiom.2024.105042_bib24",
"volume": "71",
"year": "1993"
},
{
"article-title": "Strategies for oral delivery and mitochondrial targeting of CoQ10",
"author": "Zaki",
"first-page": "1868",
"issue": "6",
"journal-title": "Drug Deliv",
"key": "10.1016/j.ebiom.2024.105042_bib25",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.1128/AAC.24.5.713",
"article-title": "Intranasal interferon as protection against experimental respiratory coronavirus infection in volunteers",
"author": "Higgins",
"doi-asserted-by": "crossref",
"first-page": "713",
"issue": "5",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ebiom.2024.105042_bib26",
"volume": "24",
"year": "1983"
},
{
"DOI": "10.1371/journal.ppat.1008737",
"article-title": "Interplay between SARS-CoV-2 and the type I interferon response",
"author": "Sa Ribero",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "PLoS Pathog",
"key": "10.1016/j.ebiom.2024.105042_bib27",
"volume": "16",
"year": "2020"
},
{
"author": "McBurney",
"first-page": "1",
"key": "10.1016/j.ebiom.2024.105042_bib28",
"series-title": "A dose ranging study of the pharmacokinetics and safety of mitoquinone administration in healthy adult volunteers",
"year": "2006"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"article-title": "Post-acute COVID-19 syndrome",
"author": "Nalbandian",
"doi-asserted-by": "crossref",
"first-page": "601",
"issue": "4",
"journal-title": "Nat Med",
"key": "10.1016/j.ebiom.2024.105042_bib29",
"volume": "27",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S235239642400077X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine"
],
"subtitle": [],
"title": "Mitoquinone mesylate as post-exposure prophylaxis against SARS-CoV-2 infection in humans: an exploratory single center pragmatic open label non-randomized pilot clinical trial with matched controls",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}