Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients
et al., Journal of Autoimmunity, doi:10.1016/j.jaut.2020.102511, NCT04317092, Nov 2020
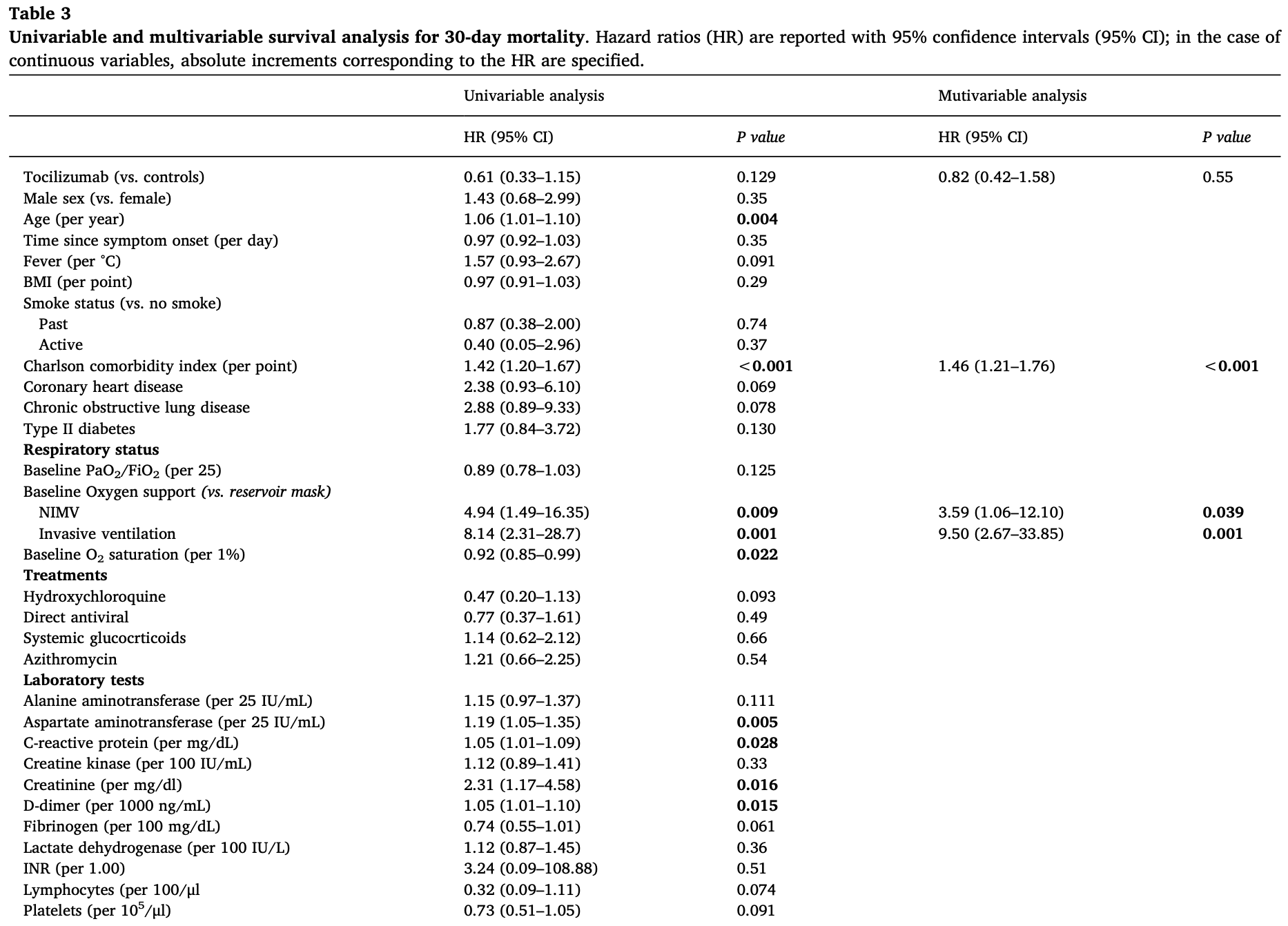
Retrospective case-control study of 128 hospitalized COVID-19 patients with severe respiratory impairment showing no significant difference in 30-day mortality with intravenous tocilizumab treatment.
|
risk of death, 18.0% lower, HR 0.82, p = 0.57, treatment 64, control 64, adjusted per study, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Canziani et al., 30 Nov 2020, retrospective, Italy, peer-reviewed, 23 authors, study period 15 March, 2020 - 22 April, 2020, trial NCT04317092 (history).
Contact: maurizio.cecconi@hunimed.eu, carlo.selmi@hunimed.eu.
Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients
Journal of Autoimmunity, doi:10.1016/j.jaut.2020.102511
A C T In cases of COVID-19 acute respiratory distress syndrome, an excessive host inflammatory response has been reported, with elevated serum interleukin-6 levels. In this multicenter retrospective cohort study we included adult patients with COVID-19, need of respiratory support, and elevated C-reactive protein who received intravenous tocilizumab in addition to standard of care. Control patients not receiving tocilizumab were matched for sex, age and respiratory support. We selected survival as the primary endpoint, along with need for invasive ventilation, thrombosis, hemorrhage, and infections as secondary endpoints at 30 days. We included 64 patients with COVID-19 in the tocilizumab group and 64 matched controls. At baseline the tocilizumab group had longer symptom duration (13 ± 5 vs. 9 ± 5 days) and received hydroxychloroquine more often than controls (100% vs. 81%). The mortality rate was similar between groups (27% with tocilizumab vs. 38%) and at multivariable analysis risk of death was not significantly influenced by tocilizumab (hazard ratio 0.61, 95% confidence interval 0.33-1.15), while being associated with the use at baseline of non invasive mechanical or invasive ventilation, and the presence of comorbidities. Among secondary outcomes, tocilizumab was associated with a lower probability of requiring invasive ventilation (hazard ratio 0.36, 95% confidence interval 0.16-0.83; P = 0.017) but not with the risk of thrombosis, bleeding, or infections. The use of intravenous tocilizumab was not associated with changes in 30-day mortality in patients with COVID-19 severe respiratory impairment. Among the secondary outcomes there was less use of invasive ventilation in the tocilizumab group.
Appendix A. Supplementary data Supplementary data to this article can be found online at https:// doi.org/10.1016/j.jaut.2020.102511 .
References
Aouba, Baldolli, Geffray, Verdon, Bergot et al., Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-217706
Aziz, Fatima, Assaly, Elevated interleukin-6 and severe COVID-19: a metaanalysis, J. Med. Virol, doi:10.1002/jmv.26085
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell
Brown, Duggal, Hou, Tidswell, Khan et al., Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among mechanically ventilated patients in the ICU: a prospective, observational study, Crit. Care Med
Brown, Grissom, Moss, Rice, Schoenfeld et al., Nonlinear imputation of pao2/fio2 from spo2/fio2 among patients with acute respiratory distress syndrome, Chest
Campochiaro, Della-Torre, Cavalli, De Luca, Ripa et al., Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study, Eur. J. Intern. Med
Cantini, Niccoli, Matarrese, Nicastri, Stobbione et al., Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact, J. Infect, doi:10.1016/j.jinf.2020.04.017
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavirritonavir in adults hospitalized with severe covid-19, N. Engl. J. Med
Cavalli, De Luca, Campochiaro, Della Torre, Roipa et al., Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheumatology
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J. Chron. Dis
Chen, Zhao, Qu, Chen, Xiong et al., Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients, Clin. Infect. Dis, doi:10.1093/cid/ciaa449
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Colaneri, Bogliolo, Valsecchi, Sacchi, Zuccaro et al., Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE), Microorganisms
Ellinghaus, Degenhardt, Bujanda, Buti, Albillos et al., Genomewide association study of severe covid-19 with respiratory failure, N. Engl. J. Med, doi:10.1056/NEJMoa2020283
Galicia, Tai, Komatsu, Shimada, Akazawa et al., Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced, Gene Immun
Geleris, Sun, Platt, Zucker, Baldwin et al., Observational study of hydroxychloroquine in hospitalized patients with covid-19, N. Engl. J. Med
Giamarellos-Bourboulis, Netea, Rovina, Akinosoglou, Antoniadou et al., Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host Microbe
Grasselli, Pesenti, Cecconi, Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response, J. Am. Med. Assoc, doi:10.1001/jama.2020.4031
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?, J. Autoimmun
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: a single center experience, J. Med. Virol
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat. Rev. Immunol
Ong, Young, Leo, Lye, Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients, Clin. Infect. Dis, doi:10.1093/cid/ciaa548
Quartuccio, Sonaglia, Mcgonagle, Fabris, Peghin et al., Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care, J. Clin. Virol
Rantala, Lajunen, Juvonen, Silvennoinen-Kassinen, Peitso et al., Association of IL-6 and IL-6R gene polymorphisms with susceptibility to respiratory tract infections in young Finnish men, Hum. Immunol
Rodríguez, Novelli, Rojas, Santis, Acosta-Ampudia et al., Autoinflammatory and autoimmune conditions at the crossroad of COVID-19, J. Autoimmun, doi:10.1016/j.jaut.2020.102506
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet
Wang, Hou, Luo, Tang, Wu et al., The laboratory tests and host immunity of COVID-19 patients with different severity of illness, JCI Insight
Xu, Han, Li, Sun, Wang et al., Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci
Yoshikawa, Hill, Yoshikawa, Popov, Galindo et al., Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection, PloS One
Zhang, Wu, Li, Zhao, Wang, The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality, Int. J. Antimicrob. Agents
Zhao, Yao, Wang, Zheng, Gao et al., A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias, Clin. Infect. Dis, doi:10.1093/cid/ciaa247
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1016/j.jaut.2020.102511",
"ISSN": [
"0896-8411"
],
"URL": "http://dx.doi.org/10.1016/j.jaut.2020.102511",
"alternative-id": [
"S0896841120301335"
],
"article-number": "102511",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Autoimmunity"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jaut.2020.102511"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Canziani",
"given": "Lorenzo M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Trovati",
"given": "Serena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brunetta",
"given": "Enrico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Testa",
"given": "Amidio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Santis",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bombardieri",
"given": "Emilio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guidelli",
"given": "Giacomo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albano",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Folci",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Squadroni",
"given": "Michela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beretta",
"given": "Giordano D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ciccarelli",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castoldi",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lleo",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aghemo",
"given": "Alessio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vernile",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malesci",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omodei",
"given": "Paolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Angelini",
"given": "Claudio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badalamenti",
"given": "Salvatore",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cecconi",
"given": "Maurizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cremonesi",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Selmi",
"given": "Carlo",
"sequence": "additional"
}
],
"container-title": "Journal of Autoimmunity",
"container-title-short": "Journal of Autoimmunity",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
8
]
],
"date-time": "2020-07-08T07:50:10Z",
"timestamp": 1594194610000
},
"deposited": {
"date-parts": [
[
2020,
10,
14
]
],
"date-time": "2020-10-14T02:37:06Z",
"timestamp": 1602643026000
},
"indexed": {
"date-parts": [
[
2025,
5,
27
]
],
"date-time": "2025-05-27T18:26:11Z",
"timestamp": 1748370371859
},
"is-referenced-by-count": 71,
"issued": {
"date-parts": [
[
2020,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
1
]
],
"date-time": "2020-11-01T00:00:00Z",
"timestamp": 1604188800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0896841120301335?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0896841120301335?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102511",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
11
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jaut.2020.102511_bib1",
"series-title": "Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins",
"year": "2020"
},
{
"article-title": "A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias",
"author": "Zhao",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.jaut.2020.102511_bib2",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/j.jaut.2020.102511_bib3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/j.jaut.2020.102511_bib4",
"volume": "395",
"year": "2020"
},
{
"article-title": "Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients",
"author": "Chen",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.jaut.2020.102511_bib5",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25948",
"article-title": "Elevated interleukin-6 and severe COVID-19: a meta-analysis",
"author": "Aziz",
"doi-asserted-by": "crossref",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.jaut.2020.102511_bib6",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25801",
"article-title": "Tocilizumab treatment in COVID-19: a single center experience",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "814",
"issue": "7",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.jaut.2020.102511_bib7",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.3390/microorganisms8050695",
"article-title": "Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE)",
"author": "Colaneri",
"doi-asserted-by": "crossref",
"journal-title": "Microorganisms",
"key": "10.1016/j.jaut.2020.102511_bib8",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104444",
"article-title": "Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care",
"author": "Quartuccio",
"doi-asserted-by": "crossref",
"journal-title": "J. Clin. Virol.",
"key": "10.1016/j.jaut.2020.102511_bib9",
"volume": "129",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.021",
"article-title": "Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study",
"author": "Campochiaro",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Eur. J. Intern. Med.",
"key": "10.1016/j.jaut.2020.102511_bib10",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30317-2",
"article-title": "Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Lancet",
"key": "10.1016/j.jaut.2020.102511_bib11",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with covid-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"issue": "25",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jaut.2020.102511_bib12",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217706",
"article-title": "Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series",
"author": "Aouba",
"doi-asserted-by": "crossref",
"journal-title": "Ann. Rheum. Dis.",
"key": "10.1016/j.jaut.2020.102511_bib13",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"first-page": "e325",
"journal-title": "Lancet Rheumatology",
"key": "10.1016/j.jaut.2020.102511_bib14",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.04.017",
"article-title": "Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact",
"author": "Cantini",
"doi-asserted-by": "crossref",
"journal-title": "J. Infect.",
"key": "10.1016/j.jaut.2020.102511_bib15",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jaut.2020.102511_bib16",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4031",
"article-title": "Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"journal-title": "J. Am. Med. Assoc.",
"key": "10.1016/j.jaut.2020.102511_bib17",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Lancet",
"key": "10.1016/j.jaut.2020.102511_bib18",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000002514",
"article-title": "Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among mechanically ventilated patients in the ICU: a prospective, observational study",
"author": "Brown",
"doi-asserted-by": "crossref",
"first-page": "1317",
"journal-title": "Crit. Care Med.",
"key": "10.1016/j.jaut.2020.102511_bib19",
"volume": "45",
"year": "2017"
},
{
"DOI": "10.1016/j.chest.2016.01.003",
"article-title": "Nonlinear imputation of pao2/fio2 from spo2/fio2 among patients with acute respiratory distress syndrome",
"author": "Brown",
"doi-asserted-by": "crossref",
"first-page": "307",
"journal-title": "Chest",
"key": "10.1016/j.jaut.2020.102511_bib20",
"volume": "150",
"year": "2016"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"article-title": "A new method of classifying prognostic comorbidity in longitudinal studies: development and validation",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "J. Chron. Dis.",
"key": "10.1016/j.jaut.2020.102511_bib21",
"volume": "40",
"year": "1987"
},
{
"DOI": "10.1093/cid/ciaa548",
"article-title": "Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients",
"author": "Ong",
"doi-asserted-by": "crossref",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.jaut.2020.102511_bib22",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105954",
"article-title": "The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "105954",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "10.1016/j.jaut.2020.102511_bib23",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2020.102506",
"article-title": "Autoinflammatory and autoimmune conditions at the crossroad of COVID- 19",
"author": "Rodríguez",
"doi-asserted-by": "crossref",
"journal-title": "J. Autoimmun.",
"key": "10.1016/j.jaut.2020.102511_bib24",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0008729",
"article-title": "Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection",
"author": "Yoshikawa",
"doi-asserted-by": "crossref",
"journal-title": "PloS One",
"key": "10.1016/j.jaut.2020.102511_bib25",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"article-title": "Imbalanced host response to SARS-CoV-2 drives development of COVID-19",
"author": "Blanco-Melo",
"doi-asserted-by": "crossref",
"first-page": "1036",
"journal-title": "Cell",
"key": "10.1016/j.jaut.2020.102511_bib26",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/sj.gene.6364120",
"article-title": "Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced",
"author": "Galicia",
"doi-asserted-by": "crossref",
"first-page": "513",
"journal-title": "Gene Immun.",
"key": "10.1016/j.jaut.2020.102511_bib27",
"volume": "5",
"year": "2004"
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"article-title": "Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "102452",
"journal-title": "J. Autoimmun.",
"key": "10.1016/j.jaut.2020.102511_bib28",
"year": "2020"
},
{
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"key": "10.1016/j.jaut.2020.102511_bib29",
"series-title": "Proc Natl Acad Sci U S A",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"article-title": "Complex immune dysregulation in COVID-19 patients with severe respiratory failure",
"author": "Giamarellos-Bourboulis",
"doi-asserted-by": "crossref",
"first-page": "992",
"issue": "6",
"journal-title": "Cell Host Microbe",
"key": "10.1016/j.jaut.2020.102511_bib30",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0353-y",
"article-title": "Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages",
"author": "Merad",
"doi-asserted-by": "crossref",
"first-page": "448",
"issue": "7",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.jaut.2020.102511_bib31",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.humimm.2010.10.010",
"article-title": "Association of IL-6 and IL-6R gene polymorphisms with susceptibility to respiratory tract infections in young Finnish men",
"author": "Rantala",
"doi-asserted-by": "crossref",
"first-page": "63",
"journal-title": "Hum. Immunol.",
"key": "10.1016/j.jaut.2020.102511_bib32",
"volume": "72",
"year": "2011"
},
{
"DOI": "10.1056/NEJMoa2020283",
"article-title": "Genomewide association study of severe covid-19 with respiratory failure",
"author": "Ellinghaus",
"doi-asserted-by": "crossref",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jaut.2020.102511_bib33",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.137799",
"article-title": "The laboratory tests and host immunity of COVID-19 patients with different severity of illness",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight",
"key": "10.1016/j.jaut.2020.102511_bib34",
"volume": "5",
"year": "2020"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0896841120301335"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "114"
}