Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study
et al., European Journal of Internal Medicine, doi:10.1016/j.ejim.2020.05.021, NCT04318366, Jun 2020
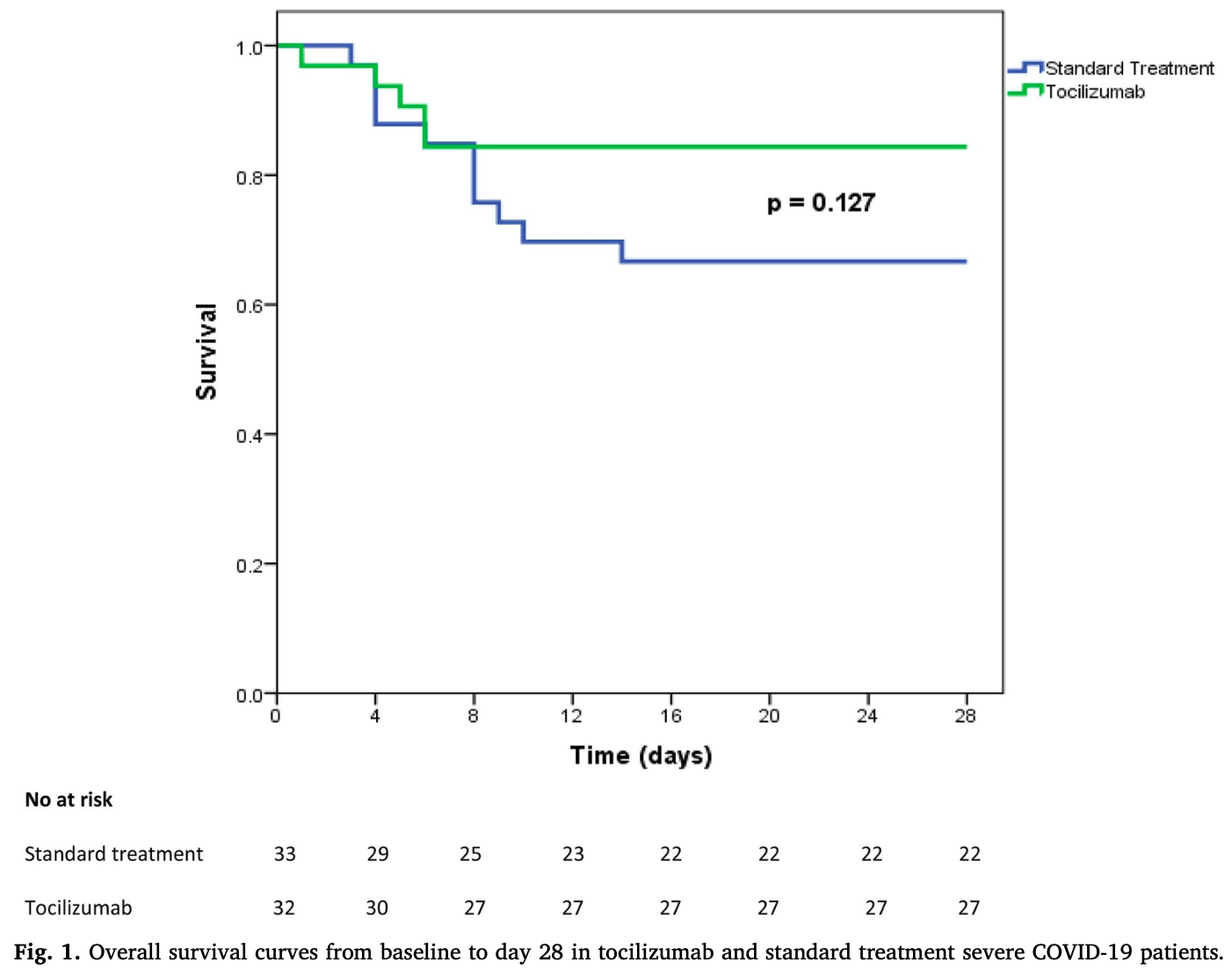
\Retrospective 65 severe COVID-19 patients showing no statistically significant differences with tocilizumab.
|
risk of death, 53.1% lower, RR 0.47, p = 0.15, treatment 5 of 32 (15.6%), control 11 of 33 (33.3%), NNT 5.6.
|
|
risk of mechanical ventilation, 106.2% higher, RR 2.06, p = 0.43, treatment 4 of 32 (12.5%), control 2 of 33 (6.1%).
|
|
risk of no hospital discharge, 27.2% lower, RR 0.73, p = 0.32, treatment 12 of 32 (37.5%), control 17 of 33 (51.5%), NNT 7.1.
|
|
risk of no improvement, 20.7% lower, RR 0.79, p = 0.61, treatment 10 of 32 (31.2%), control 13 of 33 (39.4%), NNT 12.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Campochiaro et al., 30 Jun 2020, retrospective, Italy, peer-reviewed, 16 authors, study period 13 March, 2020 - 19 March, 2020, trial NCT04318366 (history).
Contact: campochiaro.corrado@hsr.it, lorenzo.dagna@unisr.it.
Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study
European Journal of Internal Medicine, doi:10.1016/j.ejim.2020.05.021
Background: Tocilizumab (TCZ), a humanized monoclonal antibody targeting the interleukin-6 (IL-6) receptor, has been proposed for the treatment of COVID-19 patients; however, limited data are available on the safety and efficacy. Methods: We performed a retrospective study on severe COVID-19 patients with hyper-inflammatory features admitted outside intensive care units (ICUs). Patients treated with intravenous TCZ in addition to standard of care were compared to patients treated with standard of care alone. Safety and efficacy were assessed over a 28day follow-up. Results: 65 patients were included. Among them, 32 were treated with TCZ. At baseline, all patients were on high-flow supplemental oxygen and most (78% of TCZ patients and 61% of standard treatment patients) were on non-invasive ventilation. During the 28-day follow-up, 69% of TCZ patients experienced a clinical improvement compared to 61% of standard treatment patients (p = 0.61). Mortality was 15% in the tocilizumab group and 33% in standard treatment group (p = 0.15). In TCZ group, at multivariate analysis, older age was a predictor of death, whereas higher baseline PaO2:FiO2 was a predictor of clinical improvement at day 28. The rate of infection and pulmonary thrombosis was similar between the two groups. Conclusions: At day 28, clinical improvement and mortality were not statistically different between tocilizumab and standard treatment patients in our cohort. Bacterial or fungal infections were recorded in 13% of tocilizumab patients and in 12% of standard treatment patients. Confirmation of efficacy and safety will require ongoing controlled trials.
Conclusions In our study, we did not observe clear improvements in patients receiving tocilizumab compared to standard management. Infectious adverse events require careful monitoring to evaluate long-term risks. The results of ongoing randomized placebo-controlled trials are eagerly awaited to establish the role of IL-6 blockade in severe COVID-19 patients, and whether tocilizumab therapy might be safely and effectively used for treating COVID-19.
Declaration of competing interest The authors declare no conflict of interest.
References
Cao, Wang, Wen, A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N Engl J Med, doi:10.1056/nejmoa2001282
Cavalli, Luca, Campochiaro, Interleukin 1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheum, doi:10.1016/S2665-9913(20)30127-2
Ciceri, Beretta, Scandroglio, Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis, Crit Care Resusc
De Simone, Mancusi, COVID-19: Timing is Important, Eur J Intern Med, doi:10.1016/j.ejim.2020.04.019
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30120-1
Fan, Brodie, Slutsky, Acute respiratory distress syndrome advances in diagnosis and treatment, JAMA -J Am Med Assoc, doi:10.1001/jama.2017.21907
Giambenedetto, Ciccullo, Borghetti, Off-label Use of Tocilizumab in Patients with SARS-CoV-2 Infection, J Med Virol, doi:10.1002/jmv.25897
Grasselli, Pesenti, Cecconi, Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast during an Emergency Response, JAMA -J Am Med Assoc, doi:10.1001/jama.2020.4031
Grasselli, Zangrillo, Zanella, Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, JAMA, doi:10.1001/jama.2020.5394
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Kaur, Bansal, Kumar, Bansal, A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors, Bioorganic Med Chem, doi:10.1016/j.bmc.2020.115327
Le, Li, Yuan, FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome, Oncologist, doi:10.1634/theoncologist.2018-0028
Lee, Gardner, Porter, Current concepts in the diagnosis and management of cytokine release syndrome, Blood, doi:10.1182/blood-2014-05-552729
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?, J Autoimmun, doi:10.1016/j.jaut.2020.102452
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: A single center experience, J Med Virol, doi:10.1002/jmv.25801
Martins-Filho, Tavares, Santos, Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data, Eur J Intern Med, doi:10.1016/j.ejim.2020.04.043
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Pawar, Desai, Solomon, Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study, Ann Rheum Dis, doi:10.1136/annrheumdis-2018-214367
Qin, Zhou, Hu, Dysregulation of immune response in patients with COVID-19 in Wuhan, China, Clin Infect Dis, doi:10.1093/cid/ciaa248
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Sciascia, Aprà, Baffa, Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19, Clin Exp Rheumatol
Shu, Xia, Liu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med, doi:10.1016/S2213-2600(20)30079-5
Wang, Horby, Hayden, Gao, A novel coronavirus outbreak of global health concern, Lancet, doi:10.1016/S0140-6736(20)30185-9
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci, doi:10.1073/pnas.2005615117
Zangrillo, Beretta, Scandroglio, Characteristics, Treatment, Outcomes, and Cause of Death of Invasively Ventilated Patients with COVID-19 ARDS in Milan, Italy. Critical Care Resuscitation
Zangrillo, Beretta, Silvani, Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency, Crit Care Resusc
DOI record:
{
"DOI": "10.1016/j.ejim.2020.05.021",
"ISSN": [
"0953-6205"
],
"URL": "http://dx.doi.org/10.1016/j.ejim.2020.05.021",
"alternative-id": [
"S0953620520301990"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "European Journal of Internal Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ejim.2020.05.021"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 European Federation of Internal Medicine. Published by Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Campochiaro",
"given": "Corrado",
"sequence": "first"
},
{
"affiliation": [],
"family": "Della-Torre",
"given": "Emanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cavalli",
"given": "Giulio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Luca",
"given": "Giacomo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ripa",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boffini",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomelleri",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baldissera",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rovere-Querini",
"given": "Patrizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruggeri",
"given": "Annalisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monti",
"given": "Giacomo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Cobelli",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zangrillo",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tresoldi",
"given": "Moreno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castagna",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dagna",
"given": "Lorenzo",
"sequence": "additional"
}
],
"container-title": "European Journal of Internal Medicine",
"container-title-short": "European Journal of Internal Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ejinme.com",
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
5,
22
]
],
"date-time": "2020-05-22T07:34:36Z",
"timestamp": 1590132876000
},
"deposited": {
"date-parts": [
[
2024,
8,
6
]
],
"date-time": "2024-08-06T05:54:24Z",
"timestamp": 1722923664000
},
"indexed": {
"date-parts": [
[
2025,
5,
6
]
],
"date-time": "2025-05-06T18:31:49Z",
"timestamp": 1746556309108
},
"is-referenced-by-count": 327,
"issued": {
"date-parts": [
[
2020,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
1
]
],
"date-time": "2020-06-01T00:00:00Z",
"timestamp": 1590969600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0953620520301990?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0953620520301990?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "43-49",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
6
]
]
},
"published-print": {
"date-parts": [
[
2020,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30185-9",
"article-title": "A novel coronavirus outbreak of global health concern",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "470",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.1016/j.ejim.2020.05.021_bib0001",
"volume": "395",
"year": "2020"
},
{
"article-title": "An interactive web-based dashboard to track COVID-19 in real time",
"author": "Dong",
"issue": "0",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ejim.2020.05.021_bib0002",
"volume": "0",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/j.ejim.2020.05.021_bib0003",
"year": "2020"
},
{
"article-title": "Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency",
"author": "Zangrillo",
"journal-title": "Crit Care Resusc.",
"key": "10.1016/j.ejim.2020.05.021_bib0004",
"year": "2020"
},
{
"DOI": "10.1016/j.bmc.2020.115327",
"article-title": "A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors",
"author": "Kaur",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Bioorganic Med Chem",
"key": "10.1016/j.ejim.2020.05.021_bib0005",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1634/theoncologist.2018-0028",
"article-title": "FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell‐Induced Severe or Life‐Threatening Cytokine Release Syndrome",
"author": "Le",
"doi-asserted-by": "crossref",
"first-page": "943",
"issue": "8",
"journal-title": "Oncologist",
"key": "10.1016/j.ejim.2020.05.021_bib0006",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1182/blood-2014-05-552729",
"article-title": "Current concepts in the diagnosis and management of cytokine release syndrome",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "188",
"issue": "2",
"journal-title": "Blood",
"key": "10.1016/j.ejim.2020.05.021_bib0007",
"volume": "124",
"year": "2014"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.1016/j.ejim.2020.05.021_bib0008",
"volume": "395",
"year": "2020"
},
{
"article-title": "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China",
"author": "Ruan",
"journal-title": "Intensive Care Med.",
"key": "10.1016/j.ejim.2020.05.021_bib0009",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"article-title": "Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?",
"author": "Liu",
"doi-asserted-by": "crossref",
"journal-title": "J Autoimmun.",
"key": "10.1016/j.ejim.2020.05.021_bib0010",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25801",
"article-title": "Tocilizumab treatment in COVID-19: A single center experience",
"author": "Luo",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/j.ejim.2020.05.021_bib0011",
"year": "2020"
},
{
"article-title": "Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19",
"author": "Sciascia",
"journal-title": "Clin Exp Rheumatol",
"key": "10.1016/j.ejim.2020.05.021_bib0012",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"doi-asserted-by": "crossref",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.ejim.2020.05.021_bib0013",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25897",
"article-title": "Off-label Use of Tocilizumab in Patients with SARS-CoV-2 Infection",
"author": "Di Giambenedetto",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/j.ejim.2020.05.021_bib0014",
"year": "2020"
},
{
"DOI": "10.1001/jama.2017.21907",
"article-title": "Acute respiratory distress syndrome advances in diagnosis and treatment",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "698",
"issue": "7",
"journal-title": "JAMA - J Am Med Assoc",
"key": "10.1016/j.ejim.2020.05.021_bib0015",
"volume": "319",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ejim.2020.05.021_bib0016",
"year": "2020"
},
{
"key": "10.1016/j.ejim.2020.05.021_bib0017",
"unstructured": "WHO | Coronavirus disease (COVID-2019) R&D. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/. Accessed April 19, 2020."
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID-19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1033",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.ejim.2020.05.021_bib0018",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa248",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejim.2020.05.021_bib0019",
"unstructured": "Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 12]. Clin Infect Dis. 2020;ciaa248. doi:10.1093/cid/ciaa248."
},
{
"DOI": "10.1016/j.ejim.2020.04.019",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejim.2020.05.021_bib0020",
"unstructured": "de Simone G, Mancusi C. COVID-19: Timing is Important. Eur J Intern Med. 2020 Apr 13. pii: S0953-6205(20)30133-3. doi: 10.1016/j.ejim.2020.04.019. [Epub ahead of print]."
},
{
"key": "10.1016/j.ejim.2020.05.021_bib0021",
"unstructured": "U.S. National Library of Medicine, ClinicalTrials.gov(www.clinicaltrials.gov) accessed on 06 May 2020."
},
{
"article-title": "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study",
"author": "Y Shu",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.ejim.2020.05.021_bib0022",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.04.043",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejim.2020.05.021_bib0023",
"unstructured": "Martins-Filho PR, Tavares CSS, Santos VS.Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med. 2020 Apr 23. pii: S0953-6205(20)30165-5. doi: 10.1016/j.ejim.2020.04.043."
},
{
"DOI": "10.1136/annrheumdis-2018-214367",
"article-title": "Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study",
"author": "Pawar",
"doi-asserted-by": "crossref",
"first-page": "456",
"issue": "4",
"journal-title": "Ann Rheum Dis",
"key": "10.1016/j.ejim.2020.05.021_bib0024",
"volume": "78",
"year": "2019"
},
{
"article-title": "Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis",
"author": "Ciceri",
"journal-title": "Crit Care Resusc",
"key": "10.1016/j.ejim.2020.05.021_bib0025",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4031",
"article-title": "Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast during an Emergency Response",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"journal-title": "JAMA - J Am Med Assoc.",
"key": "10.1016/j.ejim.2020.05.021_bib0026",
"year": "2020"
},
{
"DOI": "10.1016/S1441-2772(23)00387-3",
"article-title": "Characteristics, Treatment, Outcomes, and Cause of Death of Invasively Ventilated Patients with COVID-19 ARDS in Milan, Italy",
"author": "Zangrillo",
"doi-asserted-by": "crossref",
"journal-title": "Critical Care Resuscitation",
"key": "10.1016/j.ejim.2020.05.021_bib0027",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin 1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Rheum",
"key": "10.1016/j.ejim.2020.05.021_bib0028",
"year": "2020"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0953620520301990"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "76"
}