Is Machine Learning a Better Way to IdentifyCOVID-19 Patients Who Might Benefit fromHydroxychloroquineTreatment?—The IDENTIFY Trial
et al., Journal of Clinical Medicine, doi:10.3390/jcm9123834, Nov 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
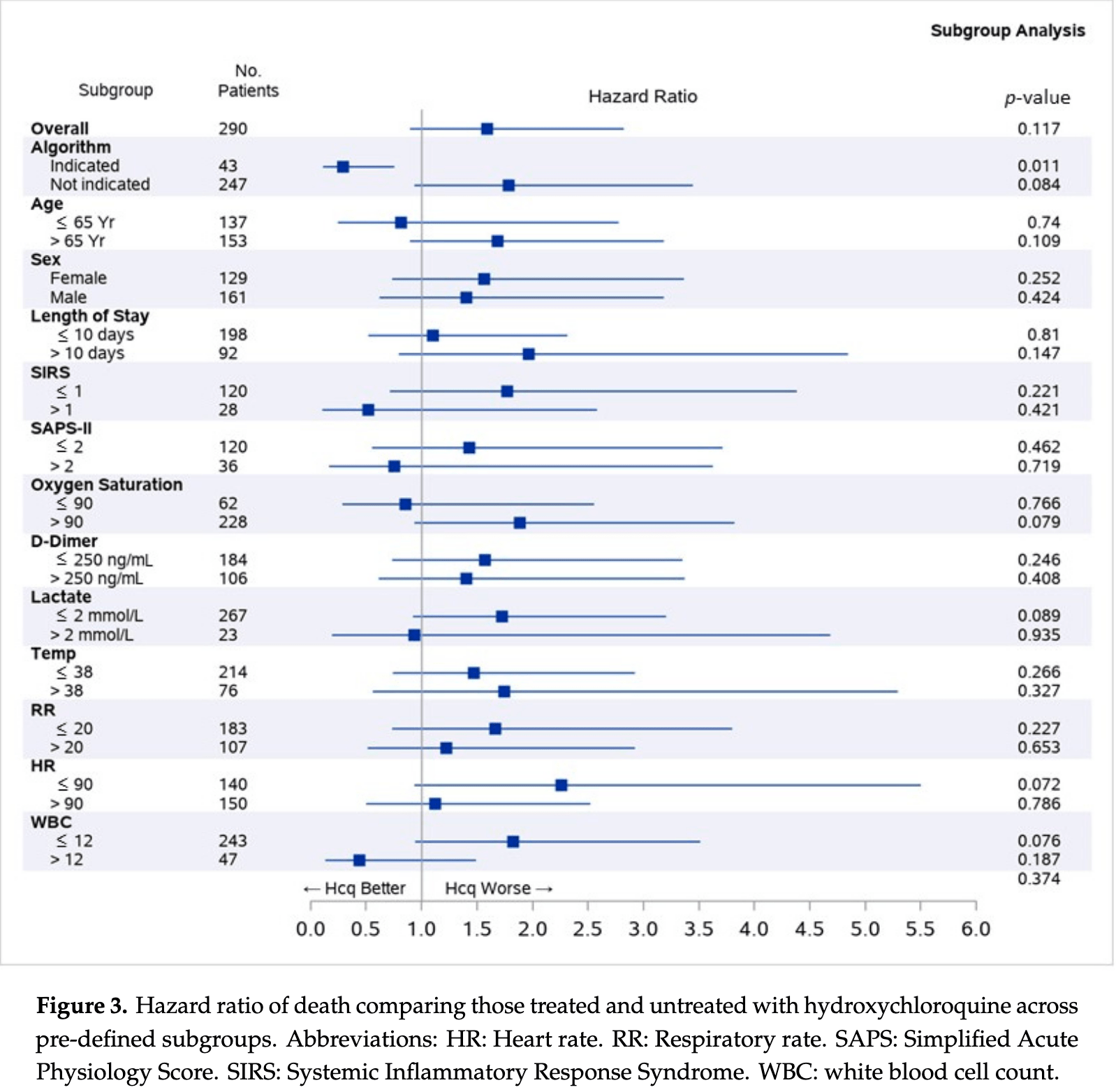
290 patient observational trial in the USA, not showing a significant difference with HCQ treatment overall, but showing significantly lower mortality in a subgroup of patients where HCQ is expected to be beneficial based on a machine learning algorithm.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 59.0% higher, HR 1.59, p = 0.12, treatment 142, control 148, adjusted per study, all patients.
|
|
risk of death, 71.0% lower, HR 0.29, p = 0.01, treatment 26, control 17, adjusted per study, subgroup of patients where treatment is predicted to be beneficial.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Burdick et al., 26 Nov 2020, prospective, USA, peer-reviewed, 14 authors.
Is Machine Learning a Better Way to Identify COVID-19 Patients Who Might Benefit from Hydroxychloroquine Treatment?—The IDENTIFY Trial
Journal of Clinical Medicine, doi:10.3390/jcm9123834
Therapeutic agents for the novel coronavirus disease 2019 (COVID-19) have been proposed, but evidence supporting their use is limited. A machine learning algorithm was developed in order to identify a subpopulation of COVID-19 patients for whom hydroxychloroquine was associated with improved survival; this population might be relevant for study in a clinical trial. A pragmatic trial was conducted at six United States hospitals. We enrolled COVID-19 patients that were admitted between 10 March and 4 June 2020. Treatment was not randomized. The study endpoint was mortality; discharge was a competing event. Hazard ratios were obtained on the entire population, and on the subpopulation indicated by the algorithm as suitable for treatment. A total of 290 patients were enrolled. In the subpopulation that was identified by the algorithm, hydroxychloroquine was associated with a statistically significant (p = 0.011) increase in survival (adjusted hazard ratio 0.29, 95% confidence interval (CI) 0.11-0.75). Adjusted survival among the algorithm indicated patients was 82.6% in the treated arm and 51.2% in the arm not treated. No association between treatment and mortality was observed in the general population. A 31% increase in survival at the end of the study was observed in a population of COVID-19 patients that were identified by a machine learning algorithm as having a better outcome with hydroxychloroquine treatment. Precision medicine approaches may be useful in identifying a subpopulation of COVID-19 patients more likely to be proven to benefit from hydroxychloroquine treatment in a clinical trial.
References
Aboughdir, Kirwin, Khader, Wang, Prognostic Value of Cardiovascular Biomarkers in COVID-19: A Review, Viruses, doi:10.3390/v12050527
Ahn, Shin, Kim, Lee, Kim et al., Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease
Alia, Grant-Kels, Does hydroxychloroquine combat COVID-19? A timeline of evidence, J. Am. Acad. Dermatol, doi:10.1016/j.jaad.2020.04.031
Aronson, Rehm, Building the foundation for genomics in precision medicine, Nat. Cell Biol, doi:10.1038/nature15816
Arshad, Kilgore, Chaudhry, Jacobsen, Wang et al., Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.06.099
Ayerbe, Risco-Risco, Ayis, The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients, Intern. Emerg. Med, doi:10.1007/s11739-020-02505-x
Azoulay, Fartoukh, Darmon, Géri, Voiriot et al., Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset, Intensiv. Care Med, doi:10.1007/s00134-020-06202-3
Biot, Daher, Chavain, Fandeur, Khalife et al., Design and Synthesis of Hydroxyferroquine Derivatives with Antimalarial and Antiviral Activities, J. Med. Chem
Bono, Ashworth, Translating cancer research into targeted therapeutics, Nat. Cell Biol, doi:10.1038/nature09339
Borba, Val, Sampaio, Alexandre, Melo et al., Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial, JAMA Netw. Open
Borba, Val, Sampaio, Alexandre, Melo et al., Effect of High vs Low Doses of Chloroquine References
Boulware, Pullen, Bangdiwala, Pastick, Lofgren et al., A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, New Engl. J. Med, doi:10.1056/NEJMoa2016638
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, New Engl. J. Med, doi:10.1056/NEJMoa2001282
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, New Engl. J. Med, doi:10.1056/NEJMoa2001282
Catteau, Dauby, Montourcy, Bottieau, Hautekiet et al., Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: A nationwide observational study of 8075 participants, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106144
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2019014
Collins, Varmus, A New Initiative on Precision Medicine, New Engl. J. Med, doi:10.1056/NEJMp1500523
Cortegiani, Ingoglia, Ippolito, Giarratano, Einav, A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19, J. Crit. Care, doi:10.1016/j.jcrc.2020.03.005
Dauby, Bottieau, The unfinished story of hydroxychloroquine in COVID-19: The right anti-inflammatory dose at the right moment?, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.10.032
Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19?, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105938
Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19?, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105938
Di Castelnuovo, Costanzo, Antinori, Berselli, Blandi et al., Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study, Eur. J. Intern. Med, doi:10.1016/j.ejim.2020.08.019
Ferner, Aronson, Chloroquine and hydroxychloroquine in Covid-19, BMJ, doi:10.1136/bmj.m1432
Fine, Gray, A Proportional Hazards Model for the Subdistribution of a Competing Risk, J. Am. Stat. Assoc, doi:10.1080/01621459.1999.10474144
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105949
Geleris, Sun, Platt, Zucker, Baldwin et al., Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2012410
Gentry, Humphrey, Thind, Hendrickson, Kurdgelashvili et al., Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: A retrospective cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(20)30305-2
Hernandez, Roman, Pasupuleti, Barboza-Meca, White, Update Alert 2: Hydroxychloroquine or Chloroquine for the Treatment or Prophylaxis of COVID, Ann. Intern. Med, doi:10.7326/L20-1054
Horby, Mafham, Linsell, Bell, Staplin et al., multi-centre, randomized, controlled trial, doi:10.1101/2020.07.15.20151852
Iyer, Hanrahan, Milowsky, Al-Ahmadie, Scott et al., Genome Sequencing Identifies a Basis for Everolimus Sensitivity, Science, doi:10.1126/science.1226344
Lai, Shih, Ko, Tang, Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105924
Lai, Shih, Ko, Tang, Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105924
Lammers, Brohet, Theunissen, Koster, Rood et al., Early hydroxychloroquine but not chloroquine use reduces ICU admission in COVID-19 patients, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.09.1460
Letai, Functional precision cancer medicine-Moving beyond pure genomics, Nat. Med, doi:10.1038/nm.4389
Mahévas, Tran, Roumier, Chabrol, Paule et al., Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data, BMJ, doi:10.1136/bmj.m1844
Menden, Iorio, Garnett, Mcdermott, Benes et al., Machine Learning Prediction of Cancer Cell Sensitivity to Drugs Based on Genomic and Chemical Properties, PLOS ONE, doi:10.1371/journal.pone.0061318
Molina, Delaugerre, Le Goff, Mela-Lima, Ponscarme et al., No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection, Méd. Mal. Infect, doi:10.1016/j.medmal.2020.03.006
Pan, Peto, Karim, Alejandria, Henao-Restrepo et al., Repurposed antiviral drugs for COVID-19-Interim WHO SOLIDARITY trial results, doi:10.1101/2020.10.15.20209817
Pintilie, Analysing and interpreting competing risk data, Stat. Med, doi:10.1002/sim.2655
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Rosenberg, Dufort, Udo, Wilberschied, Kumar et al., Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State, JAMA, doi:10.1001/jama.2020.8630
Sarma, Kaur, Kumar, Mahendru, Avti et al., Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis, J. Med Virol, doi:10.1002/jmv.25898
Schrezenmeier, Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology, Nat. Rev. Rheumatol, doi:10.1038/s41584-020-0372-x
Singh, Singh, Singh, Misra, Hydroxychloroquine in patients with COVID-19: A Systematic Review and meta-analysis, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2020.05.017
Skipper, Pastick, Engen, Bangdiwala, Abassi et al., Hydroxychloroquine in Nonhospitalized Adults With Early COVID, Ann. Intern. Med, doi:10.7326/M20-4207
Taccone, Gorham, Vincent, Hydroxychloroquine in the management of critically ill patients with COVID-19: The need for an evidence base, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30172-7
Thémans, Belkhir, Dauby, Yombi, De Greef et al., Population Pharmacokinetics of Hydroxychloroquine in COVID-19 Patients: Implications for Dose Optimization, Eur. J. Drug Metab. Pharmacokinet, doi:10.1007/s13318-020-00648-y
Tunis, Stryer, Clancy, Practical Clinical Trials, JAMA, doi:10.1001/jama.290.12.1624
Vanderweele, Ding, Sensitivity Analysis in Observational Research: Introducing the E-Value, Ann. Intern. Med, doi:10.7326/M16-2607
Voss, Hakimi, Pham, Brannon, Chen et al., Tumor Genetic Analyses of Patients with Metastatic Renal Cell Carcinoma and Extended Benefit from mTOR Inhibitor Therapy, Clin. Cancer Res, doi:10.1158/1078-0432.CCR-13-2345
Wagle, Grabiner, Van Allen, Hodis, Jacobus et al., Activating mTOR Mutations in a Patient with an Extraordinary Response on a Phase I Trial of Everolimus and Pazopanib, Cancer Discov, doi:10.1158/2159-8290.CD-13-0353
Webb, Peltan, Jensen, Hoda, Hunter et al., Clinical criteria for COVID-19-associated hyperinflammatory syndrome: A cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(20)30343-X
Yao, Ye, Zhang, Cui, Huang et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa237
Yazdany, Kim, Use of Hydroxychloroquine and Chloroquine During the COVID-19 Pandemic: What Every Clinician Should Know, Ann. Intern. Med, doi:10.7326/M20-1334
DOI record:
{
"DOI": "10.3390/jcm9123834",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm9123834",
"abstract": "<jats:p>Therapeutic agents for the novel coronavirus disease 2019 (COVID-19) have been proposed, but evidence supporting their use is limited. A machine learning algorithm was developed in order to identify a subpopulation of COVID-19 patients for whom hydroxychloroquine was associated with improved survival; this population might be relevant for study in a clinical trial. A pragmatic trial was conducted at six United States hospitals. We enrolled COVID-19 patients that were admitted between 10 March and 4 June 2020. Treatment was not randomized. The study endpoint was mortality; discharge was a competing event. Hazard ratios were obtained on the entire population, and on the subpopulation indicated by the algorithm as suitable for treatment. A total of 290 patients were enrolled. In the subpopulation that was identified by the algorithm, hydroxychloroquine was associated with a statistically significant (p = 0.011) increase in survival (adjusted hazard ratio 0.29, 95% confidence interval (CI) 0.11–0.75). Adjusted survival among the algorithm indicated patients was 82.6% in the treated arm and 51.2% in the arm not treated. No association between treatment and mortality was observed in the general population. A 31% increase in survival at the end of the study was observed in a population of COVID-19 patients that were identified by a machine learning algorithm as having a better outcome with hydroxychloroquine treatment. Precision medicine approaches may be useful in identifying a subpopulation of COVID-19 patients more likely to be proven to benefit from hydroxychloroquine treatment in a clinical trial.</jats:p>",
"alternative-id": [
"jcm9123834"
],
"author": [
{
"affiliation": [],
"family": "Burdick",
"given": "Hoyt",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8255-4423",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lam",
"given": "Carson",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3146-2243",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mataraso",
"given": "Samson",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8379-6523",
"affiliation": [],
"authenticated-orcid": false,
"family": "Siefkas",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Braden",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dellinger",
"given": "R. Phillip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCoy",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vincent",
"given": "Jean-Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Green-Saxena",
"given": "Abigail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barnes",
"given": "Gina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7745-3900",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hoffman",
"given": "Jana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calvert",
"given": "Jacob",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7614-9299",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pellegrini",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Das",
"given": "Ritankar",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
11,
27
]
],
"date-time": "2020-11-27T03:00:33Z",
"timestamp": 1606446033000
},
"deposited": {
"date-parts": [
[
2020,
11,
27
]
],
"date-time": "2020-11-27T03:12:00Z",
"timestamp": 1606446720000
},
"indexed": {
"date-parts": [
[
2024,
3,
7
]
],
"date-time": "2024-03-07T12:08:42Z",
"timestamp": 1709813322884
},
"is-referenced-by-count": 8,
"issue": "12",
"issued": {
"date-parts": [
[
2020,
11,
26
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
26
]
],
"date-time": "2020-11-26T00:00:00Z",
"timestamp": 1606348800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/9/12/3834/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3834",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2020,
11,
26
]
]
},
"published-online": {
"date-parts": [
[
2020,
11,
26
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105938",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.jcrc.2020.03.005",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1056/NEJMoa2012410",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1021/jm0601856",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1016/j.medmal.2020.03.006",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1101/2020.07.15.20151852",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1002/jmv.25898",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.dsx.2020.05.017",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1007/s11739-020-02505-x",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106144",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.ejim.2020.08.019",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.7326/M16-2607",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/S2665-9913(20)30305-2",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1001/jama.2020.8630",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1007/s00134-020-06202-3",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1007/s13318-020-00648-y",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1056/NEJMp1500523",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1038/nm.4389",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1158/1078-0432.CCR-13-2345",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1158/2159-8290.CD-13-0353",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1126/science.1226344",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1038/nature09339",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1038/nature15816",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"key": "ref30"
},
{
"DOI": "10.1001/jama.290.12.1624",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1080/01621459.1999.10474144",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/S2213-2600(20)30172-7",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1038/s41584-020-0372-x",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/j.ijid.2020.10.032",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.ijid.2020.09.1460",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"key": "ref37"
},
{
"key": "ref38"
},
{
"key": "ref39"
},
{
"DOI": "10.7326/L20-1054",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1056/NEJMoa2019014",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"key": "ref43",
"unstructured": "Global COVID-19 Prevention Trial of Hydroxychloroquine to Resumehttp://www.medscape.com/viewarticle/933174."
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1101/2020.10.15.20209817",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1136/bmj.m1432",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.jaad.2020.04.031",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.7326/M20-1334",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.4014/jmb.2003.03011",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.3390/v12050527",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1016/S2665-9913(20)30343-X",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1136/bmj.m1844",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1371/journal.pone.0061318",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1002/sim.2655",
"doi-asserted-by": "publisher",
"key": "ref55"
}
],
"reference-count": 55,
"references-count": 55,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/9/12/3834"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Is Machine Learning a Better Way to Identify COVID-19 Patients Who Might Benefit from Hydroxychloroquine Treatment?—The IDENTIFY Trial",
"type": "journal-article",
"volume": "9"
}
