Effectiveness of Colchicine for the Treatment of Long COVID
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2025.5408, CTRI/2021/11/038234, Oct 2025
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
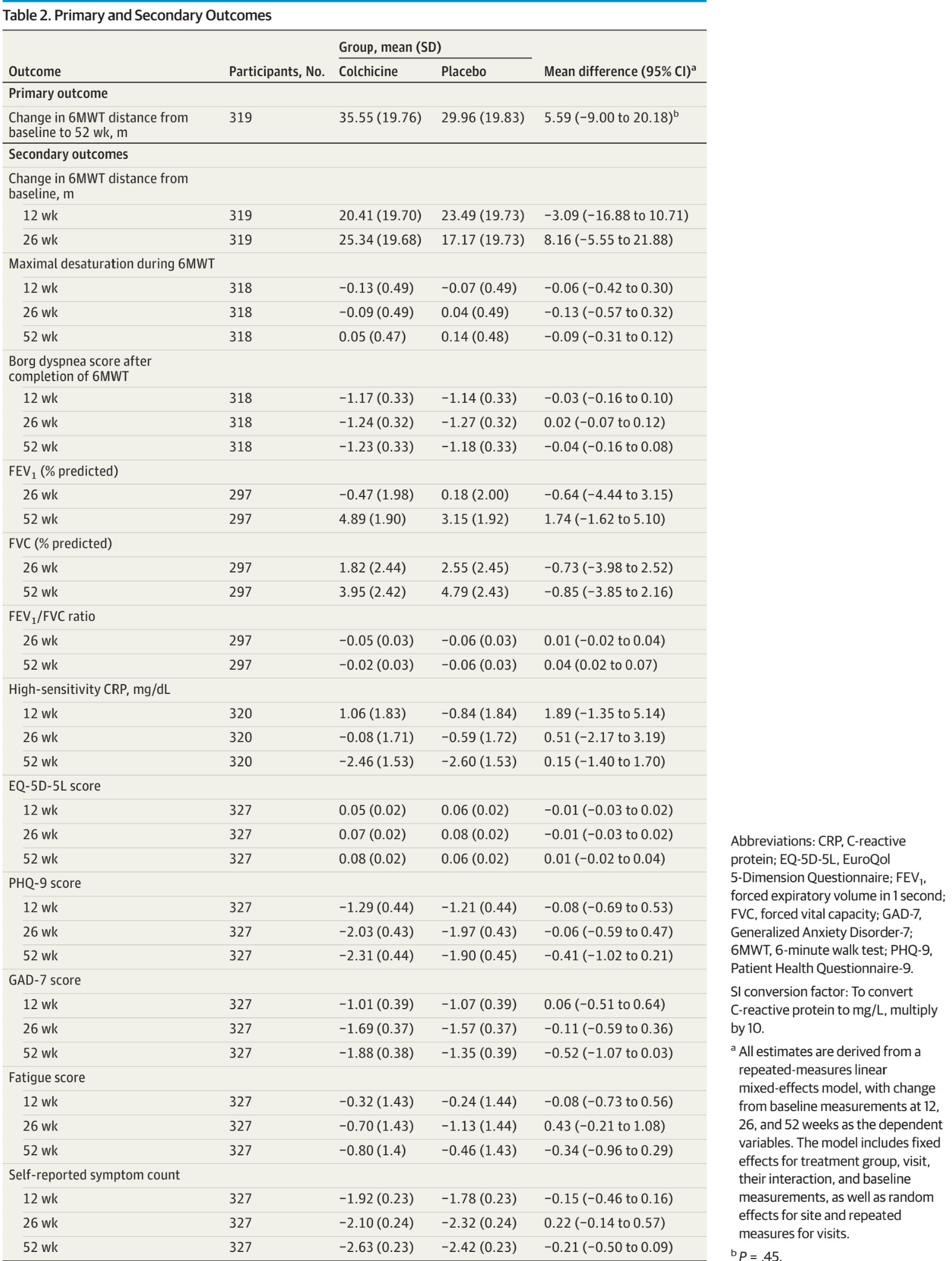
RCT 346 long COVID patients in India, showing no significant difference in functional capacity, respiratory function, or inflammatory markers with colchicine.
|
risk of long COVID, 15.7% lower, RR 0.84, p = 0.01, treatment mean 35.55 (±19.76) n=162, control mean 29.96 (±19.83) n=167, relative 6MWT improvement, day 365.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bassi et al., 20 Oct 2025, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, 22 authors, study period January 2022 - July 2023, trial CTRI/2021/11/038234.
DOI record:
{
"DOI": "10.1001/jamainternmed.2025.5408",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2025.5408",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>Long COVID is characterized by persistent symptoms after SARS-CoV-2 infection, with inflammation playing a key role in pathogenesis. Colchicine, an established anti-inflammatory agent, may reduce these symptoms by targeting inflammatory pathways.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the superiority of colchicine over placebo in improving functional outcome at 52 weeks from baseline.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>This double-blind, 1:1 randomized clinical trial recruited participants with confirmed SARS-CoV-2 infection and persistent symptoms from 8 hospitals in 6 states in India between January 2022 and July 2023. Individuals were eligible if they had functional limitation (Post–COVID-19 Functional Status scale grade 2 or more) and/or elevated inflammatory markers (high-sensitivity C-reactive protein &amp;gt;0.20 mg/dL and/or neutrophil to lymphocyte ratio &amp;gt;5). Outcomes were assessed at 12, 26, and 52 weeks after randomization. Data were analyzed from January to February 2025.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Participants were randomly assigned to receive colchicine, 0.5 mg, once or twice daily, based on body weight, or placebo for 26 weeks.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was the change in distance walked during a 6-minute walk test from baseline to 52 weeks. Secondary outcomes included changes in inflammatory markers and patient-reported outcome measures, such as quality of life, anxiety, depression, fatigue, dyspnea, measured using validated instruments.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of 346 participants included in the modified intention-to-treat analysis, 209 (60.4%) were female, 137 (39.6%) were male, and the mean (SD) age was 46 (12) years. At 52 weeks, there was no difference in mean (SD) change in 6-minute walk test distance between the colchicine and placebo groups (colchicine, 35.5 [19.76] m; placebo, 29.96 [19.83] m; mean difference, 5.59 m; 95% CI, –9.00 to 20.18; <jats:italic>P</jats:italic> = .45). Similar null findings were seen across all predefined outcomes, except for a small, nonclinically relevant difference in the mean (SD) ratio of forced expiratory volume in 1 second to forced vital capacity (colchicine, −0.02 [0.03]; placebo, −0.06 [0.03]; mean difference, 0.04; 95% CI, 0.02 to 0.07; <jats:italic>P</jats:italic> = .001).</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>In this randomized clinical trial, among adults with long COVID, colchicine did not improve functional capacity, respiratory function, or inflammatory markers. These findings underscore the need to explore alternative therapeutic approaches for long COVID.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>Clinical Trial Registry of India: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=NjE0OTQ=&amp;amp;Enc=&amp;amp;userName=\">CTRI/2021/11/038234</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Bassi",
"given": "Abhinav",
"sequence": "first"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Devasenapathy",
"given": "Niveditha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Thankachen",
"given": "Shani S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
},
{
"name": "The George Institute for Global Health, University of New South Wales, Sydney, Australia"
},
{
"name": "Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, India"
}
],
"family": "Ghosh",
"given": "Arpita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Rastogi",
"given": "Aman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Khan",
"given": "Ritika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Bahuleyan",
"given": "Bijini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
}
],
"family": "Gummidi",
"given": "Balaji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "SRM Medical College Hospital and Research Centre, Chennai, India"
}
],
"family": "Basheer",
"given": "Aneesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Community Medicine, Dr Moopen’s Medical College, Wayanad, India"
}
],
"family": "Sreelal",
"given": "Thekkumkara Prabhakaran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Medicine, Jivenrekha Multispeciality Hospital, Pune, India"
}
],
"family": "Bangi",
"given": "Ashfak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Medicine, Jivenrekha Multispeciality Hospital, Pune, India"
}
],
"family": "Shaikh",
"given": "Yasmeen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Sleep and Critical Care, All India Institute of Medical Science, Raipur, India"
}
],
"family": "Sahu",
"given": "Dibakar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Sleep and Critical Care, All India Institute of Medical Science, Raipur, India"
}
],
"family": "Rathore",
"given": "Vinay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Postgraduate Institute of Medical Education & Research, Chandigarh, India"
}
],
"family": "Bhalla",
"given": "Ashish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Postgraduate Institute of Medical Education & Research, Chandigarh, India"
}
],
"family": "Samita",
"given": "Samita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Medicine, Amrita Institute of Medical Science, Kochi, India"
}
],
"family": "Blessan",
"given": "Merlin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Medicine, Amrita Institute of Medical Science, Kochi, India"
}
],
"family": "Dipu",
"given": "T. S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Maharaja Agrasen Hospital, Jaipur, India"
}
],
"family": "Jain",
"given": "Manish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Shree Krishna Hospital, Pune, India"
}
],
"family": "Prajapati",
"given": "Abhishek Mukundbhai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiodiagnosis, All India Institute of Medical Science, Raipur, India"
}
],
"family": "Bodhey",
"given": "Narendra Kuber",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health India, Delhi, India"
},
{
"name": "The George Institute for Global Health, University of New South Wales, Sydney, Australia"
},
{
"name": "School of Public Health, Imperial College London, London, England"
},
{
"name": "Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, India"
}
],
"family": "Jha",
"given": "Vivekanand",
"sequence": "additional"
}
],
"container-title": "JAMA Internal Medicine",
"container-title-short": "JAMA Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
10,
20
]
],
"date-time": "2025-10-20T15:30:55Z",
"timestamp": 1760974255000
},
"deposited": {
"date-parts": [
[
2025,
10,
20
]
],
"date-time": "2025-10-20T15:30:56Z",
"timestamp": 1760974256000
},
"indexed": {
"date-parts": [
[
2025,
10,
20
]
],
"date-time": "2025-10-20T16:38:22Z",
"timestamp": 1760978302121,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
10,
20
]
]
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2840468/jamainternal_bassi_2025_oi_250064_1760629380.42701.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"prefix": "10.1001",
"published": {
"date-parts": [
[
2025,
10,
20
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
20
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations.",
"author": "Davis",
"doi-asserted-by": "publisher",
"first-page": "133",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "ioi250064r1",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1136/bmjopen-2023-074373",
"article-title": "Colchicine for the treatment of patients with COVID-19: an updated systematic review and meta-analysis of randomised controlled trials.",
"author": "Cheema",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "BMJ Open",
"key": "ioi250064r2",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1126/science.adl0867",
"article-title": "Solving the puzzle of Long Covid.",
"author": "Al-Aly",
"doi-asserted-by": "publisher",
"first-page": "830",
"issue": "6685",
"journal-title": "Science",
"key": "ioi250064r3",
"volume": "383",
"year": "2024"
},
{
"DOI": "10.3389/fmed.2023.1085988",
"article-title": "Biomarkers in long COVID-19: a systematic review.",
"author": "Lai",
"doi-asserted-by": "publisher",
"journal-title": "Front Med (Lausanne)",
"key": "ioi250064r4",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial.",
"author": "Bramante",
"doi-asserted-by": "publisher",
"first-page": "1119",
"issue": "10",
"journal-title": "Lancet Infect Dis",
"key": "ioi250064r5",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.bbih.2024.100733",
"article-title": "Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19.",
"author": "Isman",
"doi-asserted-by": "publisher",
"journal-title": "Brain Behav Immun Health",
"key": "ioi250064r6",
"volume": "36",
"year": "2024"
},
{
"DOI": "10.1080/23744235.2022.2043560",
"article-title": "Registered clinical trials investigating treatment of long COVID: a scoping review and recommendations for research.",
"author": "Ceban",
"doi-asserted-by": "publisher",
"first-page": "467",
"issue": "7",
"journal-title": "Infect Dis (Lond)",
"key": "ioi250064r7",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"article-title": "Colchicine–update on mechanisms of action and therapeutic uses.",
"author": "Leung",
"doi-asserted-by": "publisher",
"first-page": "341",
"issue": "3",
"journal-title": "Semin Arthritis Rheum",
"key": "ioi250064r8",
"volume": "45",
"year": "2015"
},
{
"DOI": "10.1016/j.biopha.2020.110384",
"article-title": "Colchicine prevents atrial fibrillation promotion by inhibiting IL-1ß-induced IL-6 release and atrial fibrosis in the rat sterile pericarditis model.",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Biomed Pharmacother",
"key": "ioi250064r9",
"volume": "129",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "Group",
"doi-asserted-by": "publisher",
"first-page": "1419",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "ioi250064r10",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial.",
"author": "Tardif",
"doi-asserted-by": "publisher",
"first-page": "924",
"issue": "8",
"journal-title": "Lancet Respir Med",
"key": "ioi250064r11",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3390/jcm11092615",
"article-title": "Efficacy of colchicine in the treatment of COVID-19 patients: a systematic review and meta-analysis.",
"author": "Toro-Huamanchumo",
"doi-asserted-by": "publisher",
"first-page": "2615",
"issue": "9",
"journal-title": "J Clin Med",
"key": "ioi250064r12",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2024.07.054",
"article-title": "Mechanisms of long COVID and the path toward therapeutics.",
"author": "Peluso",
"doi-asserted-by": "publisher",
"first-page": "5500",
"issue": "20",
"journal-title": "Cell",
"key": "ioi250064r13",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1186/s13063-024-08205-7",
"article-title": "Colchicine to reduce coronavirus disease-19-related inflammation and cardiovascular complications in high-risk patients post-acute infection with SARS-COV-2-a study protocol for a randomized controlled trial.",
"author": "Thankachen",
"doi-asserted-by": "publisher",
"first-page": "378",
"issue": "1",
"journal-title": "Trials",
"key": "ioi250064r14",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.1183/13993003.01494-2020",
"article-title": "The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19.",
"author": "Klok",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "ioi250064r15",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1186/s12955-021-01691-2",
"article-title": "Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19.",
"author": "Machado",
"doi-asserted-by": "publisher",
"first-page": "40",
"issue": "1",
"journal-title": "Health Qual Life Outcomes",
"key": "ioi250064r16",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1136/bmjresp-2021-001136",
"article-title": "Evaluation of the Post-COVID-19 Functional Status (PCFS) scale in a cohort of patients recovering from hypoxemic SARS-CoV-2 pneumonia.",
"author": "Benkalfate",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "BMJ Open Respir Res",
"key": "ioi250064r17",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00169-2",
"article-title": "A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study.",
"author": "Munblit",
"doi-asserted-by": "publisher",
"first-page": "715",
"issue": "7",
"journal-title": "Lancet Respir Med",
"key": "ioi250064r18",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.4103/0970-2113.125892",
"article-title": "Reference equations for 6-min walk test in healthy Indian subjects (25-80 years).",
"author": "Palaniappan Ramanathan",
"doi-asserted-by": "publisher",
"first-page": "35",
"issue": "1",
"journal-title": "Lung India",
"key": "ioi250064r19",
"volume": "31",
"year": "2014"
},
{
"DOI": "10.1111/jep.12629",
"article-title": "Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review.",
"author": "Bohannon",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "2",
"journal-title": "J Eval Clin Pract",
"key": "ioi250064r20",
"volume": "23",
"year": "2017"
},
{
"DOI": "10.1080/07853890.2022.2076901",
"article-title": "Pathophysiology and mechanism of long COVID: a comprehensive review.",
"author": "Castanares-Zapatero",
"doi-asserted-by": "publisher",
"first-page": "1473",
"issue": "1",
"journal-title": "Ann Med",
"key": "ioi250064r22",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.5498/wjp.v12.i7.874",
"article-title": "SARS-CoV-2 consequences for mental health: neuroinflammatory pathways linking COVID-19 to anxiety and depression.",
"author": "de Mello",
"doi-asserted-by": "publisher",
"first-page": "874",
"issue": "7",
"journal-title": "World J Psychiatry",
"key": "ioi250064r23",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.02.018",
"article-title": "ESCMID rapid guidelines for assessment and management of long COVID.",
"author": "Yelin",
"doi-asserted-by": "publisher",
"first-page": "955",
"issue": "7",
"journal-title": "Clin Microbiol Infect",
"key": "ioi250064r24",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41591-024-02987-8",
"article-title": "Three-year outcomes of post-acute sequelae of COVID-19.",
"author": "Cai",
"doi-asserted-by": "publisher",
"first-page": "1564",
"issue": "6",
"journal-title": "Nat Med",
"key": "ioi250064r25",
"volume": "30",
"year": "2024"
},
{
"DOI": "10.1002/jmv.28289",
"article-title": "Clinical trials on the pharmacological treatment of long COVID: a systematic review.",
"author": "Chee",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Med Virol",
"key": "ioi250064r26",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"article-title": "Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial.",
"author": "Dorward",
"doi-asserted-by": "publisher",
"first-page": "e446",
"issue": "720",
"journal-title": "Br J Gen Pract",
"key": "ioi250064r27",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00941-2",
"article-title": "Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2.",
"author": "Antonelli",
"doi-asserted-by": "publisher",
"first-page": "2263",
"issue": "10343",
"journal-title": "Lancet",
"key": "ioi250064r28",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-41879-2",
"article-title": "Long-term health impacts of COVID-19 among 242,712 adults in England.",
"author": "Atchison",
"doi-asserted-by": "publisher",
"first-page": "6588",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ioi250064r29",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1093/brain/awad377",
"article-title": "Vortioxetine for the treatment of post-COVID-19 condition: a randomized controlled trial.",
"author": "McIntyre",
"doi-asserted-by": "publisher",
"first-page": "849",
"issue": "3",
"journal-title": "Brain",
"key": "ioi250064r30",
"volume": "147",
"year": "2024"
},
{
"DOI": "10.3389/fcvm.2021.643626",
"article-title": "Vascular inflammation as a therapeutic target in COVID-19 “long haulers”: HIITing the spot?",
"author": "Christensen",
"doi-asserted-by": "publisher",
"journal-title": "Front Cardiovasc Med",
"key": "ioi250064r31",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2020.198197",
"article-title": "The role of dysregulated immune responses in COVID-19 pathogenesis.",
"author": "Tahaghoghi-Hajghorbani",
"doi-asserted-by": "publisher",
"journal-title": "Virus Res",
"key": "ioi250064r32",
"volume": "290",
"year": "2020"
},
{
"DOI": "10.1080/14756366.2021.1937144",
"article-title": "Of mitochondrion and COVID-19.",
"author": "Alfarouk",
"doi-asserted-by": "publisher",
"first-page": "1258",
"issue": "1",
"journal-title": "J Enzyme Inhib Med Chem",
"key": "ioi250064r33",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3390/metabo12100912",
"article-title": "Gut microbiota dynamics in relation to long-COVID-19 syndrome: role of probiotics to combat psychiatric complications.",
"author": "Alenazy",
"doi-asserted-by": "publisher",
"first-page": "912",
"issue": "10",
"journal-title": "Metabolites",
"key": "ioi250064r34",
"volume": "12",
"year": "2022"
},
{
"key": "ioi250064r21",
"unstructured": "Devasenapathy? N, Ghosh? A, Thankachen? SS, Bassi? A, Rastogi? A, Jha? V. SAP_Colchicine_v2.0 (21 Oct 2024).pdf. Accessed April 15, 2025. https://osf.io/bxevp"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2840468"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effectiveness of Colchicine for the Treatment of Long COVID",
"type": "journal-article"
}
