Negativización de PCR a SARS-CoV-2 en muestra respiratoria en pacientes con necesidad de asistencia recurrente
et al., Anales de Pediatría, doi:10.1016/j.anpedi.2021.01.006, Apr 2022
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 15 pediatric patients in Spain, showing faster viral clearance with HCQ+AZ, without statistical significance. Treatment time and details are not provided.
|
time to viral-, 29.2% lower, relative time 0.71, p = 0.45, treatment median 17.0 IQR 16.0 n=5, control median 24.0 IQR 21.0 n=5, onset to clearance.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bassets-Bosch et al., 30 Apr 2022, retrospective, Spain, peer-reviewed, 5 authors, study period 11 March, 2020 - 30 April, 2020, this trial uses multiple treatments in the treatment arm (combined with AZ) - results of individual treatments may vary.
Contact: cristinalarrosaespinosa@gmail.com, segonzalez@vhebron.net, sgonzalezperis@gmail.com.
Abstract: Anales de Pediatría 96 (2022) 350---371
Figura 1
A) Imagen de dermatoscopia: lesión seudovascular. B) Lesión cupuliforme, irregular, de coloración eritematosa viva.
Bibliografía
1. Stefanaki C, Chardalias L, Soura E, Katsarou A, Stratigos
A. Paediatric melanoma. J Eur Acad Dermatology Venereol.
2017;31:1604---15.
2. Merkel EA, Mohan LS, Shi K, Panah E, Zhang B, Gerami P.
Paediatric melanoma: clinical update, genetic basis, and advances in diagnosis. Lancet Child Adolesc Heal. 2019;3:646---54,
http://dx.doi.org/10.1016/S2352-4642(19)30116-6.
3. Lu C, Zhang J, Nagahawatte P, Easton J, Lee S, Liu
Z, et al. The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol. 2015;135:816---23,
http://dx.doi.org/10.1038/jid.2014.425.
4. Requena C, Rubio L, Traves V, Sanmartín O, Nagore E, Llombart
B, et al. Fluorescence in situ hybridization for the differential
diagnosis between Spitz naevus and spitzoid melanoma. Histopathology. 2012;61:899---909.
5. Wiesner T, Kutzner H, Cerroni L, Jr MJM, Klaus J, Murali R, et al.
Genomic aberrations in spitzoid tumours and their implications
for diagnosis, prognosis and therapy. 2017;48:113---31.
6. Stefanaki C, Stefanaki K, Antoniou C, Argyrakos T, Patereli A,
Stratigos A, et al. Cell cycle and apoptosis regulators in Spitz
Negativización de PCR a SARS-CoV-2
en muestra respiratoria en pacientes
con necesidad de asistencia
recurrente
SARS-CoV-2 PCR negativization in respiratory
sample in patients with need for recurring
assistance
Sra. Editora:
Además de las consecuencias en términos de morbimortalidad (especialmente en pacientes adultos), la pandemia de
nevi: Comparison with melanomas and common nevi. J Am Acad
Dermatol. 2007;56:815---24.
Cristina Larrosa a,∗ , Antonio Torrelo b , Luis Madero a
y Álvaro Lassaletta a
a
Unidad de Onco-Hematología Pediátrica y Trasplante de
Progenitores Hematopoyéticos, Hospital Universitario Niño
Jesús, Madrid, España
b
Unidad de Dermatología, Hospital Universitario Niño
Jesús, Madrid, España
∗
Autor para correspondencia.
Correo electrónico: cristinalarrosaespinosa@gmail.com
(C. Larrosa).
https://doi.org/10.1016/j.anpedi.2021.01.008
1695-4033/ © 2021 Publicado por Elsevier España, S.L.U. en
nombre de Asociación Española de Pediatrı́a. Este es un artı́culo
Open Access bajo la licencia CC BY-NC-ND (http://
creativecommons.org/licenses/by-nc-nd/4.0/).
SARS-CoV-2/COVID-19 ha amenazado con bloquear los circuitos asistenciales habituales. En este sentido, la detección
de SARS-CoV-2 en pacientes con necesidad de contacto reiterado con el sistema sanitario ha obligado a establecer
estrategias seguras de control clínico y de negativización.
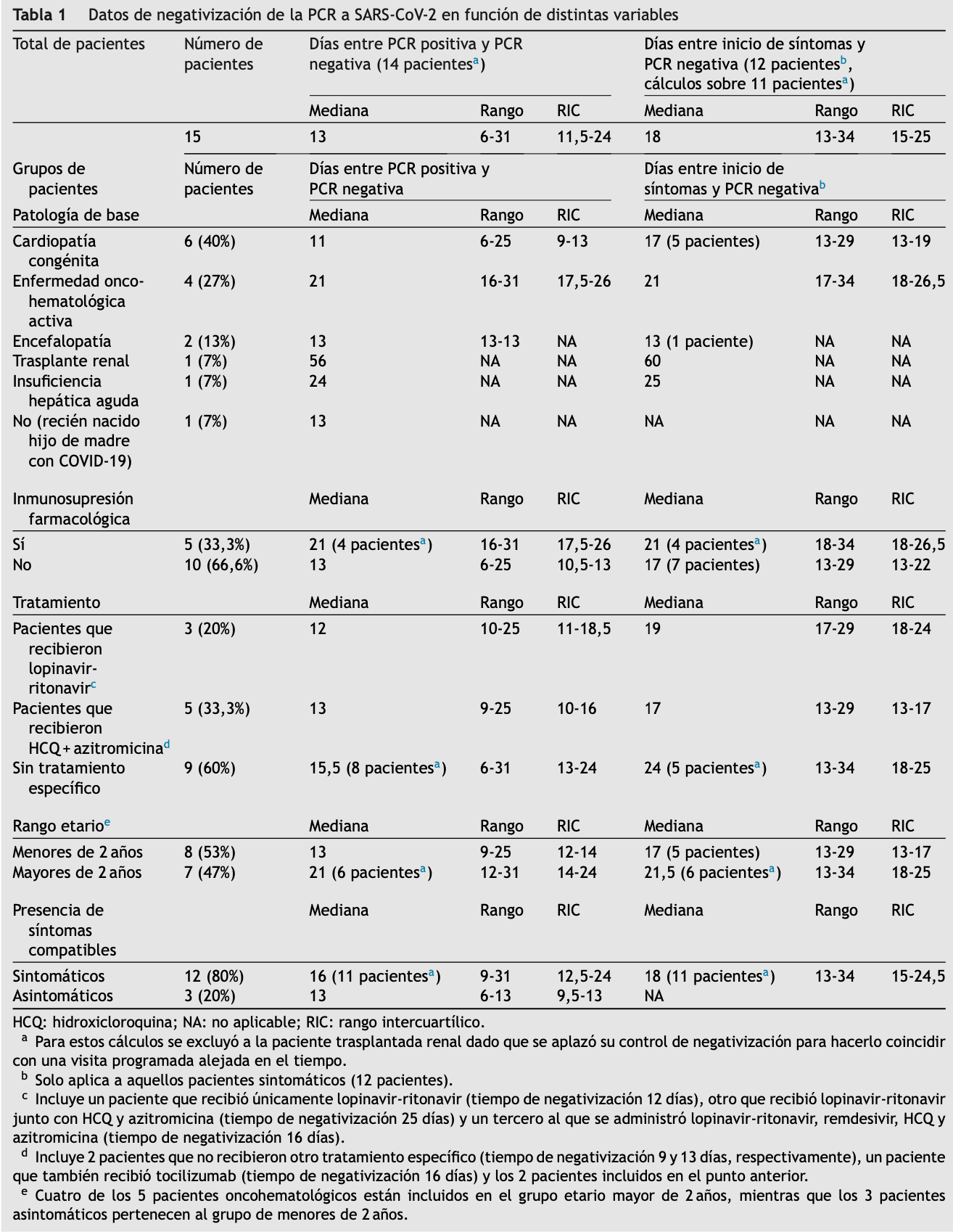
Se analizaron los datos de los pacientes diagnosticados
de infección por SARS-CoV-2 mediante PCR en muestra respiratoria entre el 11 de marzo y el 30 de abril de 2020 en
un hospital terciario pediátrico de referencia de Barcelona
(España), a los que se hubiera practicado posteriormente
un nuevo estudio para comprobar la negativización de la
PCR en aspirado nasofaríngeo. Dicho estudio se realizó a
pacientes con necesidad de asistencia seriada en hospitales de día, procedimientos, hospitalizaciones programadas,
etc. Para estos controles clínicos y de negativización, y pos357
CARTAS CIENTÍFICAS
Tabla 1
Datos de negativización de la PCR a SARS-CoV-2 en función de distintas variables
Total de pacientes
Grupos de
pacientes
Número..
DOI record:
{
"DOI": "10.1016/j.anpedi.2021.01.006",
"ISSN": [
"1695-4033"
],
"URL": "http://dx.doi.org/10.1016/j.anpedi.2021.01.006",
"alternative-id": [
"S1695403321000126"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Negativización de PCR a SARS-CoV-2 en muestra respiratoria en pacientes con necesidad de asistencia recurrente"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Anales de Pediatría"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.anpedi.2021.01.006"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Asociación Española de Pediatría. Published by Elsevier España, S.L.U."
}
],
"author": [
{
"affiliation": [],
"family": "Bassets-Bosch",
"given": "Alba",
"sequence": "first"
},
{
"affiliation": [],
"family": "Raya-Muñoz",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wörner-Tomasa",
"given": "Núria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Melendo-Pérez",
"given": "Susana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González-Peris",
"given": "Sebastià",
"sequence": "additional"
}
],
"container-title": "Anales de Pediatría",
"container-title-short": "Anales de Pediatría",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.es",
"elsevier.es",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
1,
27
]
],
"date-time": "2021-01-27T12:55:37Z",
"timestamp": 1611752137000
},
"deposited": {
"date-parts": [
[
2022,
4,
25
]
],
"date-time": "2022-04-25T22:12:38Z",
"timestamp": 1650924758000
},
"indexed": {
"date-parts": [
[
2022,
4,
25
]
],
"date-time": "2022-04-25T22:41:09Z",
"timestamp": 1650926469644
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "es",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T00:00:00Z",
"timestamp": 1648771200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
27
]
],
"date-time": "2021-01-27T00:00:00Z",
"timestamp": 1611705600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1695403321000126?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1695403321000126?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "357-359",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
4
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1136/bmj.m1443",
"article-title": "Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "m1443",
"journal-title": "BMJ.",
"key": "10.1016/j.anpedi.2021.01.006_bib0030",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa351",
"article-title": "Factors associated with prolonged viral RNA shedding in patients with Coronavirus Disease 2019 (COVID-19)",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "799",
"journal-title": "Clin Infect Dis.",
"key": "10.1016/j.anpedi.2021.01.006_bib0035",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1097/INF.0000000000002814",
"article-title": "Duration of respiratory and gastrointestinal viral shedding in children with SARS-CoV-2: A systematic review and synthesis of data",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "e249",
"journal-title": "Pediatr Infect Dis J.",
"key": "10.1016/j.anpedi.2021.01.006_bib0040",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa619",
"article-title": "To interpret the SARS-Cov-2 test consider the cycle threshold value",
"author": "Tom",
"doi-asserted-by": "crossref",
"first-page": "2252",
"journal-title": "Clin Infect Dis.",
"key": "10.1016/j.anpedi.2021.01.006_bib0045",
"volume": "71",
"year": "2020"
},
{
"key": "10.1016/j.anpedi.2021.01.006_bib0050",
"unstructured": "Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19 [actualizado 19 Oct 2020]. Disponible en: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1695403321000126"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pediatrics, Perinatology and Child Health"
],
"subtitle": [],
"title": "Negativización de PCR a SARS-CoV-2 en muestra respiratoria en pacientes con necesidad de asistencia recurrente",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "96"
}
