Recent Hydroxychloroquine Use Is Not Significantly Associated with Positive PCR Results for SARS-CoV-2: A Nationwide Observational Study in South Korea
et al., Viruses 2021, doi:10.3390/v13020329, Feb 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
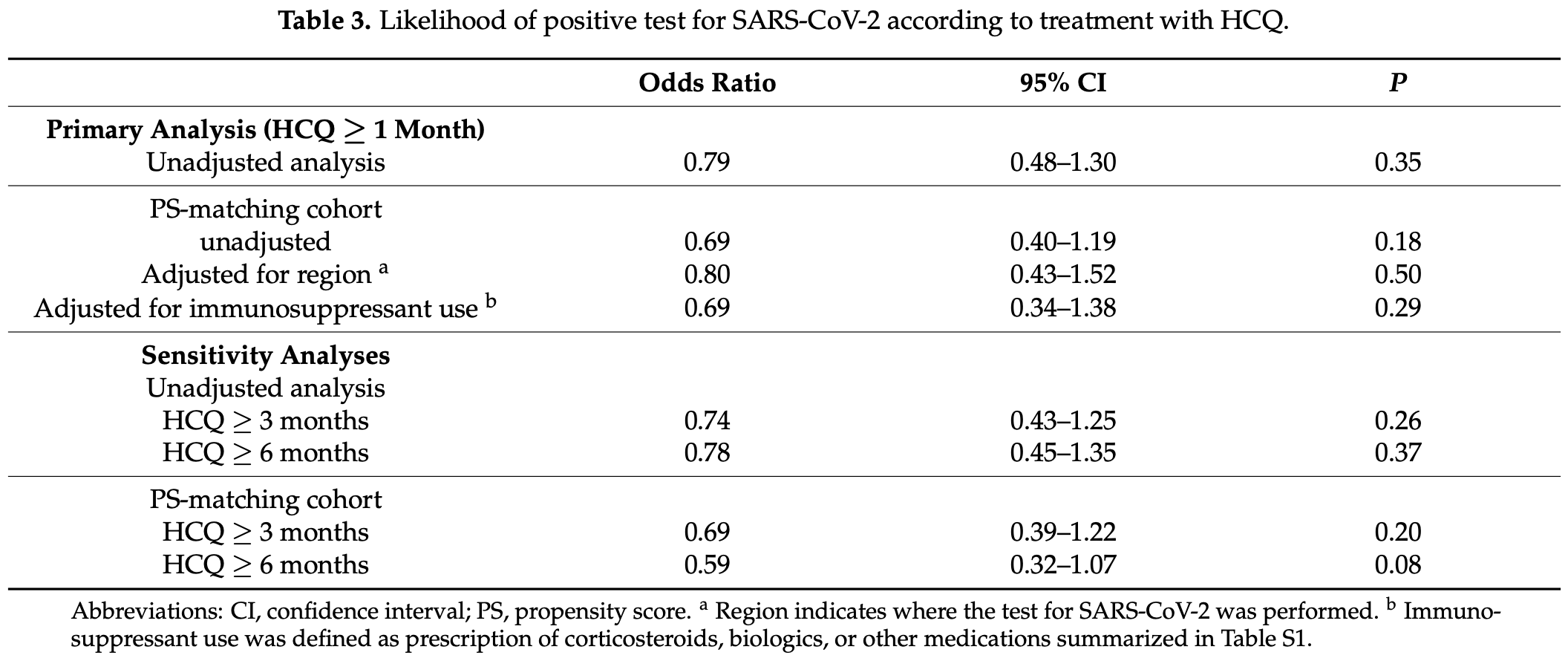
Retrospective database analysis of prior HCQ usage in South Korea, showing non-statistically significantly lower mortality and cases with treatment.
Although the 30% fewer cases is not statistically significant, it is consistent with the significant 29% fewer cases [21‑36%] from meta-analysis of the 82 cases results to date.
|
risk of case, 30.3% lower, RR 0.70, p = 0.18, treatment 16 of 743 (2.2%), control 91 of 2,698 (3.4%), NNT 82, odds ratio converted to relative risk, PSM.
|
|
risk of case, 19.5% lower, RR 0.81, p = 0.50, treatment 16 of 743 (2.2%), control 91 of 2,698 (3.4%), odds ratio converted to relative risk, PSM, adjusted for region.
|
|
risk of case, 30.3% lower, RR 0.70, p = 0.30, treatment 16 of 743 (2.2%), control 91 of 2,698 (3.4%), NNT 82, odds ratio converted to relative risk, PSM, adjusted for immunosuppresant use.
|
|
risk of case, 40.2% lower, RR 0.60, p = 0.09, odds ratio converted to relative risk, PSM, HCQ ≥6 months.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bae et al., 20 Feb 2021, retrospective, propensity score matching, South Korea, peer-reviewed, 8 authors.
Recent Hydroxychloroquine Use Is Not Significantly Associated with Positive PCR Results for SARS-CoV-2: A Nationwide Observational Study in South Korea
Viruses, doi:10.3390/v13020329
Background: To evaluate the role of hydroxychloroquine (HCQ) as pre-exposure prophylaxis against coronavirus disease 2019 (COVID-19), we investigated the prevalence of positive test results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing according to recent HCQ use in patients who had been tested using nationwide health-insurance data of South Korea. Methods: All adults tested for SARS-CoV-2 from 20 January 2020 to 15 May 2020 were identified. HCQ users were defined as patients who had been pretreated with HCQ for at least 30 days until the date of SARS-CoV-2 testing. The prevalence of positive PCR results for SARS-CoV-2 was compared between HCQ users and nonusers. Results: Of a total of 216,686 individuals who had been tested for SARS-CoV-2, 743 (0.3%) were pretreated with HCQ. The prevalence of positive results was not significantly different between HCQ users (2.2%) and nonusers (2.7%; P = 0.35), with an odds ratio of 0.79 (95% confidence interval (CI), 0.48-1.30). Propensity score-matched-cohort analysis showed similar results in terms of the prevalence of positive results (2.2% in HCQ users vs. 3.1% in nonusers; P = 0.18), with an odds ratio of 0.69 (95% CI, 0.40-1.19). The rate of positive PCR was not significantly different in long-term HCQ users (more than 3 or 6 months) compared with nonusers. Conclusions: In this population-based study, recent exposure to HCQ was not significantly associated with a lower risk of SARS-CoV-2 infection. Our data do not support the use of HCQ as pre-exposure prophylaxis against COVID-19.
Supplementary Materials: The following are available online at https://www.mdpi.com/1999-491 5/13/2/329/s1, Table S1 . Diagnostic codes and drug codes used in this study.
Conflicts of Interest: The authors have no potential conflicts of interest.
References
Abella, Jolkovsky, Biney, Uspal, Hyman et al., Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial, JAMA Intern. Med, doi:10.1001/jamainternmed.2020.6319
Ahn, Kang, Seo, Choe, Song et al., A Case of Breakthrough COVID-19 during Hydroxychloroquine Maintenance, J. Korean Med. Sci, doi:10.3346/jkms.2020.35.e231
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19-Preliminary Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Boulware, Pullen, Bangdiwala, Pastick, Lofgren et al., A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2016638
Chorin, Wadhwani, Magnani, Dai, Shulman et al., QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin, Heart Rhythm, doi:10.1016/j.hrthm.2020.05.014
Cipriani, Zorzi, Ceccato, Capone, Parolin et al., Arrhythmic profile and 24-hour QT interval variability in COVID-19 patients treated with hydroxychloroquine and azithromycin, Int. J. Cardiol, doi:10.1016/j.ijcard.2020.05.036
Gao, Tian, Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci. Trend, doi:10.5582/bst.2020.01047
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105949
Hung, Lung, Tso, Liu, Chung et al., Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An openlabel, randomised, phase 2 trial, Lancet, doi:10.1016/S0140-6736(20)31042-4
Kupferschmidt, Cohen, Race to find COVID-19 treatments accelerates, Science, doi:10.1126/science.367.6485.1412
Lahouati, Mériglier, Martin, Bouchet, Desclaux et al., COVID-19 infection also occurs in patients taking hydroxychloroquine, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa193
Lima, Brito, Overhage, Nizer, The potential of drug repositioning as a short-term strategy for the control and treatment of COVID-19 (SARS-CoV-2): A systematic review, Arch. Virol, doi:10.1007/s00705-020-04693-5
Mahévas, Tran, Roumier, Chabrol, Paule et al., Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data, BMJ
Martinez, Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus, Antimicrob. Agents Chemother, doi:10.1128/AAC.00399-20
Mavrikakis, Papazoglou, Sfikakis, Vaiopoulos, Rougas, Retinal toxicity in long term hydroxychloroquine treatment, Ann. Rheum. Dis, doi:10.1136/ard.55.3.187
Mercuro, Yen, Shim, Maher, Mccoy et al., Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus Disease 2019 (COVID-19), JAMA Cardiol, doi:10.1001/jamacardio.2020.1834
Monti, Balduzzi, Delvino, Bellis, Quadrelli et al., Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-217424
Parks, Smith, How to Discover Antiviral Drugs Quickly, N. Engl. J. Med, doi:10.1056/NEJMcibr2007042
Peck, Early diagnosis and rapid isolation: Response to COVID-19 outbreak in Korea, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2020.04.025
Rajasingham, Bangdiwala, Nicol, Skipper, Pastick et al., Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: A randomized trial, Clin. Infect. Dis, doi:10.1093/cid/ciaa1571
Schrezenmeier, Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology, Nat. Rev. Rheumatol, doi:10.1038/s41584-020-0372-x
Tan, Magill, Parise, Arguin, Doxycycline for malaria chemoprophylaxis and treatment: Report from the CDC expert meeting on malaria chemoprophylaxis, Am. J. Trop. Med. Hyg, doi:10.4269/ajtmh.2011.10-0285
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial, BMJ
Vincent, Bergeron, Benjannet, Erickson, Rollin et al., Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol. J, doi:10.1186/1743-422X-2-69
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Yao, Ye, Zhang, Cui, Huang et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa237
DOI record:
{
"DOI": "10.3390/v13020329",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v13020329",
"abstract": "<jats:p>Background: To evaluate the role of hydroxychloroquine (HCQ) as pre-exposure prophylaxis against coronavirus disease 2019 (COVID-19), we investigated the prevalence of positive test results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing according to recent HCQ use in patients who had been tested using nationwide health-insurance data of South Korea. Methods: All adults tested for SARS-CoV-2 from 20 January 2020 to 15 May 2020 were identified. HCQ users were defined as patients who had been pretreated with HCQ for at least 30 days until the date of SARS-CoV-2 testing. The prevalence of positive PCR results for SARS-CoV-2 was compared between HCQ users and nonusers. Results: Of a total of 216,686 individuals who had been tested for SARS-CoV-2, 743 (0.3%) were pretreated with HCQ. The prevalence of positive results was not significantly different between HCQ users (2.2%) and nonusers (2.7%; P = 0.35), with an odds ratio of 0.79 (95% confidence interval (CI), 0.48–1.30). Propensity score-matched-cohort analysis showed similar results in terms of the prevalence of positive results (2.2% in HCQ users vs. 3.1% in nonusers; P = 0.18), with an odds ratio of 0.69 (95% CI, 0.40–1.19). The rate of positive PCR was not significantly different in long-term HCQ users (more than 3 or 6 months) compared with nonusers. Conclusions: In this population-based study, recent exposure to HCQ was not significantly associated with a lower risk of SARS-CoV-2 infection. Our data do not support the use of HCQ as pre-exposure prophylaxis against COVID-19.</jats:p>",
"alternative-id": [
"v13020329"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6375-3657",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bae",
"given": "Seongman",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7284-4964",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ghang",
"given": "Byeongzu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Ye-Jee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2738-5882",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lim",
"given": "Joon Seo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yun",
"given": "Sung-Cheol",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8029-7355",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Yong-Gil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1381-8787",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Sang-Oh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6596-8253",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Sung-Han",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
22
]
],
"date-time": "2021-02-22T04:59:31Z",
"timestamp": 1613969971000
},
"deposited": {
"date-parts": [
[
2021,
2,
26
]
],
"date-time": "2021-02-26T13:13:45Z",
"timestamp": 1614345225000
},
"funder": [
{
"award": [
"HW20C2062"
],
"name": "Ministry of Health & Welfare, Republic of Korea"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
28
]
],
"date-time": "2023-08-28T17:31:42Z",
"timestamp": 1693243902664
},
"is-referenced-by-count": 4,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
20
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2021,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
20
]
],
"date-time": "2021-02-20T00:00:00Z",
"timestamp": 1613779200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/13/2/329/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "329",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
2,
20
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
20
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMcibr2007042",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1128/AAC.00399-20",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1126/science.367.6485.1412",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1007/s00705-020-04693-5",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1186/1743-422X-2-69",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.5582/bst.2020.01047",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"article-title": "Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data",
"author": "Mahévas",
"journal-title": "BMJ",
"key": "ref12",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1849",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1038/s41584-020-0372-x",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.4269/ajtmh.2011.10-0285",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1136/annrheumdis-2020-217424",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1093/jac/dkaa193",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.3346/jkms.2020.35.e231",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1001/jamainternmed.2020.6319",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1093/cid/ciaa1571",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1136/ard.55.3.187",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/j.ijcard.2020.05.036",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.hrthm.2020.05.014",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1001/jamacardio.2020.1834",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.cmi.2020.04.025",
"doi-asserted-by": "publisher",
"key": "ref26"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/13/2/329"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "Recent Hydroxychloroquine Use Is Not Significantly Associated with Positive PCR Results for SARS-CoV-2: A Nationwide Observational Study in South Korea",
"type": "journal-article",
"volume": "13"
}
